
1. THE NEED FOR A "GREENER" REVOLUTION
One of the outstanding scientific accomplishments of the twentieth century has been the extraordinary success of agricultural research in providing numerous developing nations with the agricultural tools necessary to dramatically increase staple food production (e.g., cereals). This "green revolution" was responsible for staving off famine in many countries by providing the energy and protein required to sustain a rapidly growing world population (see Fig. 1). For example, in Sub-Saharan Africa, in the Near East/North Africa, and in South Asia food provisions (in terms of dietary energy supply per capita per day) have kept pace with or exceeded population growth in those regions (see Fig. 2). Unfortunately, this commendable agricultural accomplishment resulted in unforseen nutritional problems for nearly half of the world's population, especially pregnant women, infants and children (United Nations ACCSN, 1992).
Micronutrient(2) malnutrition is now a massive and rapidly growing public health problem among nearly all poor people in many developing nations (see discussion below) affecting about 40% of the world's people (Buyckx, 1993; Ramalingaswami, 1995). The rapid increase in people afflicted with micronutrient malnutrition during the last three decades coincides with the expansion of "green revolution" cropping systems in developing nations. This pernicious, but preventable human health crisis calls for a new agenda for agriculture, an agenda that no longer focuses the agricultural community only on staple food production as the primary goal, but one that also recognizes the urgent need for agriculture to adjure attention to producing enough food of high nutritional quality and diversity to satisfy a balanced diet for all people thereby insuring healthy and productive lives. Global food systems must be changed in ways that will insure that balanced nutrient supplies are available continuously to all people in adequate and affordable amounts (Combs et al., 1997a; Combs, et al., 1996; Welch et al., 1997b).
The apparent consequences of "green revolution" cropping systems on micronutrient malnutrition are clearly demonstrated in several world regions. For example, in South Asia, the introduction of modern wheat and rice production practices (which resulted in about a 200% increase rice production and a 400% increase in wheat production over the past 30 years) is associated with time trends in the growth of iron deficiency anemia among non-pregnant, premenopausal women, and negatively related to time trends in the iron density (mg Fe per kcal of available food) of diets (Figure 3). The same types of negative associations are also found in data collected from China, Sub-Saharan Africa, South America, Middle America/Caribbean, and Southeast Asia (United Nations ACCSN, 1992).
What are the causal factors responsible for this worrisome trend in global nutritional health, and has agriculture unintendedly contributed to massive increases in micronutrient malnutrition globally? There is no way of knowing with any certainty, but certain changes in crop production systems could be contributing significantly to the growing problem of micronutrient malnutrition (i.e., "hidden hunger").
The use of modern cereal cropping systems in many developing nations has been paralleled by decreased per capita production of traditional foods having higher micronutrient density (e.g., legume seeds and pulses). This has resulted in lower availability of foods rich in micronutrients for the poor at least in some regions of the world. For example, in Bangladesh between the years of 1978 and 1996, per capita rice and wheat production kept pace with or exceeded population growth while total pulse production dramatically declined during this entire period (see Figure 4). Obviously, those agricultural changes that occurred in Bangladesh during the past three decades have resulted in increased rice and wheat production, but at the expense of pulse and legume production. The actual reasons responsible for this trend are not known, but they may be related to government policies and subsidies that promoted the expansion of cereal crops without considering the effects of such policies on pulse and legume production.
Interestingly, as eaten, legume seeds are richer sources of micronutrients in diets then are cereal grains for two reasons. First, most commonly eaten pulse seeds (beans, lentils, chickpeas, peas, etc.) contain higher concentrations of micronutrients when compared to whole rice and wheat grain (Table 1). Second, pulse seeds are normally prepared and eaten without removal of seed parts while both rice and wheat grain are normally processed (i.e., milled and/or polished) before cooking and consumption which removes various grain parts (e.g., the germ and aleurone layer) that are rich sources of micronutrients (see Table 2). Thus, a combination of decreased availability and consumption of pulses, and an increase in consumption of the less micronutrient-rich milled rice and other refined cereal grain products could be contributing to the growth in micronutrient malnutrition seen in many nations of the developing world.
The consequences of micronutrient deficiencies are profound and insidious (discussed below). They diminish the health, livelihood, and well being of all those afflicted and the productivity and stability of the societies in which these people live in ways that are not always obvious (Combs et al., 1996; Welch et al., 1997b). Clearly, agriculture's commendable struggle to end hunger globally coincides with a rapid growth of "hidden hunger" among the world's poor - a clear case of a failing global food system.
Apparently, agriculture's primary focus on producing more cereals rich in calories to meet growing energy demands from population pressures has resulted in reduced food supplies high in micronutrients, especially for the world's poor. We are now faced with a growing world population that is not starving in great numbers from energy deprivation, but that is becoming more malnourished with every passing day. The magnitude and profound consequences of micronutrient malnutrition to human health and well-being demands a new "greener" revolution. This new revolution should explicitly link agricultural production to human nutrition and health with the goal of eliminating "hidden hunger" globally and providing dietary nutritional balance to all in sustainable ways.
2. GLOBAL TRENDS IN MICRONUTRIENT MALNUTRITION
Micronutrient malnutrition has become a global problem of immense proportions (United Nations ACCSN, 1992). . Incredibly, over 2 billion people, mostly poor women, infants and children in the world, are now iron, iodine and/or vitamin A deficient (Mason and Garcia, 1993). Most certainly, several other micronutrients, including zinc, selenium and other vitamins (e.g., riboflavin, vitamin C, and vitamin B12), are limiting in the diets of vast numbers of people in many regions of the world (although little is known about the actual extent of these deficiencies because of technical and clinical problems in determining their distribution globally) (World Health Organization, 1996).
The world's nations have made fighting "hidden hunger" a high international priority (Anonymous, 1992; Anonymous, 1995; Anonymous, 1996; Underwood, 1998). Furthermore, certain micronutrient deficiencies (especially Fe deficiency) are an issue even in developed countries such as the United States (Frazao, 1996; Welch et al., 1997b). The following discussion documents the astounding magnitude of "hidden hunger" and diet-related diseases in both developing and developed nations.
2.1. Developing nations
The World Health Organization (WHO) of the United Nations has documented the extent of certain micronutrient deficiencies (including Fe, vitamin A and I) in humans in the developing world (United Nations ACCSN, 1992). Figure 5 depicts the distribution of world regions where populations have been or are at high risk of developing iron, vitamin A and iodine deficiencies. Table 3 shows the world populations affected by, or at risk of developing micronutrient malnutrition. The total number of people affected is truly astounding (2 billion people)! Clearly, something must be done to overcome this world crisis in nutrition and health.
Other micronutrient deficiencies including, Se and Zn, are also known to affect significant numbers of people over large areas in some countries. Keshan disease is a Se-responsive endemic cardiomyopathy (heart disease) that predominately affects children and pre-menopausal women in certain areas of China (Ge and Yang, 1993). Kashin-Beck disease is an endemic osteoarthropathy (bone disease) that has also been linked to inadequate-Se intakes in people within certain areas of the former Soviet Union and in China. This disease primarily affects children between 5 and 13 years of age. Both of these Se deficiency-related diseases may be the result of low available Se status of the soils in the regions affected which is responsible for the low Se content of the food eaten in these regions (World Health Organization, 1996).
Recently, Se has been linked to I deficiency disorders because of Se's role in thyroid hormone metabolism as a constituent of iodothyronine 5'-deiodinase (Foster, 1993). Thus, in areas where I and Se deficiencies exist concurrently, I fortification programs must also include adequate Se in order for the I to be utilized by people deficient in both nutrients (Yi and Xia, 1997).
Zinc deficiency among women, infants and children may be as widespread globally as iron deficiency because the dietary sources of the most bioavailable(3) Fe (i.e., animal meats) are also the most important sources of bioavailable Zn in peoples' diets (Gibson, 1994; Shrimpton, 1993). A diet which results in Fe deficiency, therefore, is likely to also predispose an individual to Zn deficiency. Unfortunately, there is no reliable clinical diagnostic criteria available to determine Zn status in humans accurately. For this reason the prevalence of Zn deficiency in the world is still uncertain, but its prevalence is presumed to be very extensive among many poor pregnant women, infants and children in many developing countries. In many countries when Zn supplements are given to poor children, growth rates increase demonstrating that Zn deficiency may be widespread (Gibson, 1994). For example, in Guatemala, Rivera et al. (1998) studied the impact of Zn supplementation on the growth and body composition of infants in a community-based, double-blind intervention trial. Stunted infants, receiving the Zn supplement, gained 1.40 cm more in height than stunted children given the placebo during the study. They concluded that Zn supplementation of these rural Guatemalan infants during 6.9 months enhanced the linear growth of those who were stunted at baseline demonstrating Zn deficiency in this population group.
Similar results have been reported for increased growth rates of infants and children from low-income families receiving Zn supplementation in studies carried out in Ecuador, Chile, Bangladesh, Iran, Turkey, and even in the United States, Canada and France where Zn supplementation increased growth rates. These finding suggest that Zn deficiency may be widespread globally.
Interestingly, recent studies in some developing countries have reported that Zn supplements can reduce the severity and frequency of diarrhea in infants and children (Hambidge, 1992; Sazawal et al., 1996). If this phenomenon is substantiated and found to be widespread, then Zn status will become a public health issue of great importance to the developing world because childhood diarrhea is an immense public health problem in many countries.
2.2. Developed nations
Developed nations, in comparison to developing nations, have much lower percentages of their populations affected by micronutrient malnutrition. However, there are still significant proportions of certain population groups within developed nations where micronutrient malnutrition persists and is a public health issue. This is especially true for Fe deficiency and for I deficiency disorders in developed countries that do not iodize their salt (Hetzel, 1990). For example, in the United States Fe deficiency among low-income children between the ages of one and two (21%) and anemia among black, low-income, pregnant women (41%) is remarkable high (see Table 4) (Looker et al., 1997). In the United States, the 1988 Surgeon General's report stated that the magnitude of Fe deficiency in children, adolescents and women of childbearing age was of special concern to public health officials (U.S. Department of Health and Human Services, 1988). Recent studies (Looker et al., 1997) report that Fe deficiency and Fe deficiency anemia in toddlers, adolescent girls, women of childbearing age, and in some ethnic groups are still relatively common in the United States.
As stated previously, the extent of Zn deficiency in developed countries, as in developing countries, is not known. However, there are several reports concerning certain population groups in North America that suggest that Zn deficiency may be a prevalent problem of significant magnitude in this world region. For example, in an intervention trial in Birmingham, Alabama, Goldenberg and coworkers (1995) reported that poor, black pregnant women supplemented with 25 mg Zn per day delivered infants with significantly greater birth weights and head circumferences than women receiving a placebo. Still other studies have reported that marginal Zn deficiency (as indicated by low plasma Zn levels and rapid plasma Zn disappearance rates) to be quite common among the elderly and among pre-menopausal women in the United States. Additionally, several studies have shown that low Zn intakes are growth-limiting in some otherwise healthy infants, pre-school children and adolescents in both the United States and Canada (Gibson et al., 1991; Hambidge, 1997).
While Ca is not considered to be a micronutrient, deficiencies of Ca do impact the health and well being of millions of mostly elderly people throughout the world. Osteoporosis is a progressive demineralization of bone to which chronic sub-optimal Ca intakes contributes. It mostly affects post menopausal women during their later life. Ironically, it is preventable with proper diet and exercise, but requires that adequate sources of Ca are present in people's diets throughout all stages of life from fetal development, infancy, childhood, adolescence, middle age and old age. This diet-related disease alone costs the United States economy about $13 to $18 billion annually in healthcare expenditures (Barefield, 1996).
There are known interactions of Ca with other trace elements that can lead to bone disease in people. For example, B is a newly discovered trace element that appears to play a role in calcium homeostasis and in bone formation in humans (Culver and Hubbard, 1996; Nielsen, 1994). Interestingly, some reports suggest that B may be limiting in the diets of some elderly people living in the United States, thereby possibly implicating low-B dietary intakes to poor Ca utilization and osteoporosis in this population group (Chapin et al., 1997; Nielsen, 1996; Nielsen, 1997).
It is becoming clearer that Se is another example of a micronutrient where normal dietary intakes are not high enough to meet optimal health needs for many people in developed countries. A recent study has shown that increased Se intake from supplements can reduce the risk of various cancers in humans. Clark et al. (1996) (in a double-blind study with data collected from 1,312 individuals over a 10 year period from subjects given either a 200 µg Se supplement or a placebo) reported that the Se-treated subjects had much lower rates of various kinds of cancer when compared to those subjects given a placebo. The Se group showed a 37% reduction in all cancer incidence and a 50% reduction in cancer mortality. There were 63% fewer prostate cancers, 58% fewer colon-rectal cancers and 46% fewer lung cancers in those receiving the Se supplement compared to those receiving the placebo. The base dietary intakes of Se by both groups exceeded the USA's recommended Se intake of 55-70 µg Se per day. Apparently, increasing Se intakes above the level required by humans to satisfy normal nutritional demands helped reduce the incidence of certain types of cancer.
These are only a few examples where dietary micronutrient inadequacies are reducing the health and well being of nearly half the world's population. It clearly demonstrates that agriculturalist should become concerned about sustainable solutions to "hidden hunger" in both developing and developed nations. Not to do so is socially and morally unacceptable.
CONSEQUENCES OF MICRONUTRIENT MALNUTRITION
Micronutrient malnutrition not only affects the health, well being and livelihood of all those individuals and families afflicted, but it also adversely impacts programs to control population growth, societal stability and national development efforts as depicted in Figure 6. What are these adverse consequences of micronutrient malnutrition on individuals, families, economies, societies, and nations? The following discussion presents some examples of the detrimental consequences of "hidden hunger" on these aspects of human existence.
3.1 Health Consequences
In infants and young children, micronutrient malnutrition diminishes their motivation and curiosity thereby reducing their exploratory activities including their time at play. Consequently, micronutrient malnutrition impairs mental and cognitive development by reducing interactions of children with their environment, their peers and their care providers. Micronutrient malnourished children, deprived of their genetic potential for mental and physical development, become adults with lower intellectual and physical abilities less able to provide for themselves and/or their families. Often they are less productive with higher rates of chronic disease and disabilities. The economic loss to some nations resulting from micronutrient malnutrition can be as high as 5% of their gross national product in lost lives, disabilities, and lower labor productivity. For example, in Bangladesh and India, a total of $18 billion was calculated to be lost from their economies as a result of malnutrition (Islam and Tori, 1998).
In expectant mothers, micronutrient malnutrition leads to varying degrees of mental retardation in their offspring. Low-birth weight infants are know to have intelligent quotients (IQs) that average about 5 points lower then infants born with weights within the normal range. Children who are severely stunted for their age have IQs from 5 to 10 points lower then their non-stunted peers. Infants delivered by women who are malnourished and underweight are also underweight and small which increases their mortality risk. Not surprisingly , because the nutritional status of pregnant and lactating women, infants and children has such dramatic adverse effects on mental, physical and social development, the progression of developing societies is largely dependent on the nutritional health of expectant women, infants and children.
During periods of rapid growth (i.e., fetal development, infancy, childhood, adolescents) demands for micronutrient are greatest and intakes should increased or else growth failure or deficiency diseases develop. During these growth stages, micronutrient deficiency symptoms are most prevalent. Further, interactions between micronutrient malnutrition and infectious diseases are very important. These interactions can confound efforts to control various diseases. For example, vitamin A deficiency interacts with measles synergistically and increases the severity of measles and leads to vitamin A-deficiency-blindness and death. Iron deficiency anemia is often associated with malnutrition, malaria, and hookworm infection. While it is difficult to attribute micronutrient deficiencies directly to many diseases, it is also clear that prevention of micronutrient deficiencies can contribute to infectious disease control (Ramalingaswami, 1995).
Individuals with extreme iron deficiency anemia are at high risk of tissue hypoxia and heart failure which can lead to death in iron-deficient young children and pregnant women. Indeed, maternal anemia aggravated by blood loss during child birth is responsible for most of the maternal mortality in the world with 20% of all maternal deaths being attributed to Fe deficiency anemia (Maberly et al., 1994) and 30% of children who enter hospitals with severe anemia die (Anonymous, 1994). Infants born to mothers that are iron deficiency anemic are commonly stunted and unhealthy. Iron deficiency is associated with poor attention span, inadequate fine motor skills, and reduced memory retention in children (Walter et al., 1997). Some data suggests that iron deficiency in women during pregnancy may cause irreversible damage to fetal brain development leading to permanent impairment of intellectual development in the offspring (Gordon, 1997). In Chile, researchers have reported that Fe-deficient infants have learning disabilities later in school even if the Fe deficiency conditions is corrected early on. Iron deficiency is associated with infant prematurity and low birth weight; this can result in lingering frailties such as immune system dysfunction and growth failure (McGuire, 1993).
Both physical performance and work productivity are significantly reduced in Fe-deficient individuals. For example, plantation workers in Indonesia, cotton mill workers in China and workers in Sri Lanka when provided Fe supplements increased their work productivity from 20 to 50% (Anonymous, 1994; Li et al., 1994; Maberly et al., 1994). In Bangladesh, an economic study (funded by the U.S. Agency for International Development) reported that a two-thirds reduction in iron deficiency anemia would result in a $3.2 billion benefit to the agricultural gross domestic product within six years as a result of increased labor productivity by reducing iron deficiency anemia among workers (Sanghvi, 1996). Indeed, some economic studies suggest that the calculated benefit/cost ratio from giving iron supplements to anemic workers is potentially as high as 260:1. In food-Fe fortification studies it was reported that the value of benefits, in terms of labor output, from fortification was 7, 42, and 70 times the cost of the fortification programs in field trials in Indonesia, Kenya and Mexico, respectively (Sanghvi, 1996).
Deficiencies of Fe, vitamin A and I are all associated with increased mortality rates among mostly poor women, infants and children. For example, severe vitamin A deficiency results in a fatality rate of 60% among those afflicted. Even moderate vitamin A deficiency is associated with a 23% increase in infant and children mortality in regions where vitamin A deficiency is prevalent (McGuire, 1993).
Vitamin A deficiency is the most important cause of childhood blindness in developing nations. Even before vitamin A deficiency impairs the visual system in children, the integrity of epithelial barriers and the immune system are compromised leading to increased severity of certain infections and increased risk of childhood death. Vitamin A deficiency is endemic in 39 countries based on clinical eye signs, symptoms, or deficient vitamin A blood levels (<0.35 µmol L-1). Including subclinical vitamin A deficiency (blood levels < 0.70 µmol L-1) increases the number of nations having significant numbers of people affected to 60 with an additional 13 countries suspected of having significant numbers of subclinical vitamin A deficiencies among children (World Health Organization, 1995).
High rates (about 0.5 to 1%) of neonatal deaths and still births are associated with maternal I deficiency in regions were people have suboptimal I intakes. Additionally, severe I deficiency in pregnant women leads to irreversible mental retardation and neurological disorders (cretinism) in their progeny. Other I deficiency disorders (IDD) include deafness, muteness, and mild to moderate mental retardation which are all irreversible. They limit children's ability to learn, their educational attainment, their occupational choices and ultimately, their future livelihoods and welfare. In countries were IDD is prevalent, there is an immense cost to societies in terms of lost human potential and its economic ramifications.
Acute Zn deficiency in humans is rare. It results in hypogonadism and dwarfism in men, growth retardation, orificial and acral dermatitis, diarrhea, and alopecia. Impaired reproductive performance and difficulty in parturition are also associated with Zn deficiency (Prasad, 1993; Prasad, 1996; World Health Organization, 1996). However, mild Zn deficiency may be widespread, but unreported globally because there is no established clinical method for determining marginal Zn deficiency in humans (Endre et al., 1990; Larsen, 1997; Reinhold, 1988; Shrimpton, 1993). The consequences of mild Zn deficiency include reduced growth rates in infants and children, impaired resistance to infection, impaired taste acuity and delayed wound healing. Additionally, evidence is accumulating that mild Zn deficiency impairs brain function and behavioral development in children (Penland, 1997).
Past evidence suggests that Zn plays a critical role as an antioxidant and in antioxidant defense systems, but the exact mechanism that Zn plays is unknown. Recently, Kim et al. (1998) reported that marginal Zn deficiency lowers the lymphatic absorption of vitamin E (-tocopherol) in rats. Their results demonstrate that the intestinal absorption of vitamin E is reduced by low Zn status. Thus, Zn nutriture may have a profound effect on the bioavailability of lipid soluble vitamins like vitamin E. This provides at least one role of Zn in antioxidant mechanisms through its promotion of vitamin E absorption, a vitamin well known to act as an antioxidant in humans.
In China, regional Se deficiency resulted in a human disease designated Keshan disease (named for the Keshan region of China where the incidence of the disease is extensive) . The disease mainly affected children and pre-menopausal women causing cardiomyopathy with multifocal myocardial necrosis without affecting the coronary arteries. Treatment of the disease with Se therapy after establishment is of little or no value. Today, the disease is not considered to be a major problem as a result of Se intervention (including supplementation) programs in China.
Recently, the interaction between I and Se has been receiving attention because of Se's role in thyroid hormone metabolism (as a constituent of iodothyronine 5'-deiodinase). Combined deficiencies of Se and I may have adverse effects on infant growth, development and survival. Selenium deficiency could also be a problem in areas where I deficiency disorders are not clearly related to I intakes and where the diet is also low in available Se (Hofbauer et al., 1997; World Health Organization, 1996).
3.2. Societal and Development Consequences
Linear conceptualizing can lead to the belief that improving the nutritional health of people would result in increased population growth rates, a consequence that would bode poorly for current attempts to reduce population growth rates globally to address dwindling natural resources and increased environmental pollution problems. However, history suggests that this is not the case. Those countries that have dramatically reduced the incidence of malnutrition are also those countries that have reduced their birth rates the most over the last century (such as Japan and other industrialized countries). There is a growing body of evidence that improved nutritional health promotes reductions in the birth rate in the long term (Behrman, 1993; Sanghvi, 1996). This is especially true for micronutrients that are known to play critical roles in brain development during fetal growth and in cognitive ability during infancy and childhood (e.g., Fe, I, Zn, B). Allowing micronutrient malnutrition to go unchecked in a society leads to more unhealthy people, reduced labor productivity, lower educational attainments in children, reduced school enrollments and attendance, increased morbidity and mortality, lower standards of living, higher health care costs and civil discontent. This is a formula for governmental instability. Dietary adequacies of these nutrients are required for optimum health and for maximizing the expression of cognitive functions in offspring. Providing adequate nutrition to pregnant women, infants and children can lead to better school attendance and higher educational attainment, and ultimately, higher-paying jobs , later marriages and improved worker productivity- all outcomes that enhance civilization. Providing adequate nutrition to all increases the opportunities for increased family income, decreased family size, a higher standard of living, and societal contentment. Thus, eliminating micronutrient malnutrition should be a high priority goal for many developing nations. Achieving it would contribute greatly to economic growth by directly enhancing health and labor productivity, and indirectly by improving cognitive achievement in all individuals within a society. Over time, nations that provide adequate nutrition for their population are better able to reduce their birth rates to desirable levels (Welch et al., 1997). Therefore, adequate nutrition is an indispensable, but often overlooked component of individual and family health, livelihood and well being, as well as an important factor in sustainable national development efforts and in population control.
In a review of the economic rationale for investing in nutrition in developing countries Behrman (1993) concluded that improving nutritional health contributes in important ways to the advancement of national development goals. These goals included growth in labor productivity and in the distribution of benefits resulting from such growth among societal members. Improving nutritional health contributes effectively by tending to favor the poorest population groups. Thus, investing in programs to improve nutrition is a cost effective use of national resources because it dramatically reduces the enormous social and financial losses caused by poor health resulting from malnutrition. Investing in better human nutrition programs enhances investments in other sectors including health, education, and agriculture.
CHANGING PARADIGMS: LINKING AGRICULTURAL PRODUCTION TO HUMAN NEEDS
Adequate and balanced nutrient output from agricultural systems has never been an explicit goal of agricultural enterprises or of national, state or local government policies. The current paradigm of agriculture is directed at increasing production per unit of land at a lower cost to the producer without concern for the nutritional quality of the products produced or for the nutrient output of the systems they employ. Lately, many agriculturalists have become concerned about the effects of their practices on the environment and efforts have been made to make agriculture more responsive to environmental concerns. Regrettably, most of the global human nutrition community has taken a medical approach to eliminating micronutrient malnutrition by viewing "hidden hunger" as a disease that must be "treated". Thus, most intervention programs use micronutrient supplements or food fortification programs to "treat" micronutrient malnutrition. While many of these types of nutritional interventions prove successful in the short term, none have proven to be sustainable over the long term. Indeed, many programs become ineffectual after a few years because of numerous social, governmental and technical problems (including problems with logistics and sustained funding; diminished political interest and commitment, and decreased interest and reduced dedication of the people administering the programs) (Beaton et al., 1993; Welch et al., 1997b; Yip, 1997). Unfortunately, most international nutrition programs directed at eliminating micronutrient malnutrition do not consider using agriculture as a primary weapon in their arsenal against this public health crisis even though their policy leaders at the highest levels have recognized the importance of agriculture to sustainable nutrition programs (Anonymous, 1992).
Clearly, making balanced and adequate nutrient output and nutritional quality explicit goals of agriculture are greatly needed if we are to find sustainable solutions to micronutrient malnutrition. Furthermore, having the nutrition community use agriculture as a primary tool in addressing micronutrient malnutrition is long overdue. Without forging a close link between agriculture and nutrition, eliminating micronutrient malnutrition globally in sustainable ways will not likely occur, and "hidden hunger" will continue to grow as a debilitating and destabilizing force in the world.
ESSENTIAL MICRONUTRIENTS FOR HUMANS: DIETARY NEEDS AND SOURCES
Currently, their are at least 15 trace elements (i.e., micronutrient elements) and 12 vitamins(4) known to be essential for human life. Undoubtedly, other micronutrients will be discovered to be essential in the future. A deficiency in any one of these essential nutrients will have adverse effects on human health, livelihood and well being. If widespread within a population, deficiencies of any micronutrient can diminish economic growth, societal stability, and national development. Assuring dietary diversity and food abundance for all are currently the most advantages ways of providing optimum nutrition. The more diverse the sources of food in a diet, the more likely that adequate and balanced amounts of all micronutrients will be consumed (Anonymous, 1993). Interventions using micronutrient supplements and food fortificants are important programs currently being used to provide adequate micronutrient nutrition in many nations. These programs are very important in treating micronutrient deficiencies to large numbers of people relatively quickly; they should continue to be used as short- to medium-term interventions for decreasing micronutrient malnutrition. However, agriculture should become a primary intervention tool if we are to eliminate "hidden hunger" in sustainable ways (Welch et al., 1997b). Unfortunately, in many nations, introduction of high yielding cereal crops and trends to less heterogeneous farming systems has resulted in reduced diversity of food available to low-income individuals and families, and therefore, decreased access to and increased cost of more diverse food sources in the market place especially for the poor. As stated previously, this trend of less food diversity could be a contributing factor in the spread of micronutrient malnutrition among poor women, infants and children in developing nations.
5.1. Essential Trace Elements
The known trace elements (As, B, Cr, Cu, F, I, Fe, Mn, Mo, Ni, Se, Si, V, and Zn), their functions, deficiency symptoms, dietary needs and sources of foods rich in each are given in Table 5. Because of space limitations, Table 5 lists the estimated daily requirements for each trace element for adult males only. For additional information on daily requirements for women, infants and children refer to (Abdulla et al., 1993; Brown, 1990; National Research Council, 1989; Wenlock, 1992; World Health Organization, 1996).
Other potentially essential trace elements include Al, Cd, Li, Pb, and Sn (Nielsen, 1990; Nielsen, 1997). These elements were not included in Table 5 because they have not been conclusively proven to be essential for humans even though some evidence of their beneficial effects on higher animals has been reported. More in-depth information concerning essential trace elements can be found in various review articles (Abdulla et al., 1993; Nielsen, 1990; Nielsen, 1992; Nielsen, 1993; Nielsen, 1997; Wenlock, 1992; Wharton, 1992; World Health Organization, 1996 and in Mertz et al. 1986; 1987).
5.2. Vitamins
Dietary deficiencies of vitamins result in specific abnormalities because of the derangement of particular metabolic processes. Currently, there are at least 13 vitamins known to be essential for human life. These include 9 water soluble vitamins and four fat soluble vitamins. Table 6 list each and summarizes some of their deficiency symptoms, biological functions, dietary requirements and foods that contain high levels of each vitamin. The requirements listed are for adult males because of space limitations (see Brown, 1990; National Research Council, 1989).
Vitamins are classified as either fat soluble or water soluble because of their different solubilities in non polar (fats) and polar (water) solvents. Their solubility characteristics have implications for their bioavailability from foods to humans with the fat soluble vitamins requiring the presence of adequate levels of dietary fats to be absorbed from the gut while the water soluble vitamins do not require fats to be absorbed by intestinal mucosal cells from the gastrointestinal tract.
Other food-derived organic compounds ("quasi-vitamins") not listed in Table 6 may also function as vitamins, but their human requirements are not known even though they are associated with important biological activities. For example, myo-inositol, choline, taurine, carnitine, pyrroloquinoline quinone, ubiquinones, orotic acid, bioflavonoids, p-aminobenzoic acid, and lipoic acid are known to be essential, or play important functions in some higher animals, but they have not been proven to be essential for normal humans (Combs, 1992; National Research Council, 1989). For more detailed information concerning vitamins and "quasi-vitamins" in foods and their functions refer to the following references: Brown, 1990; Combs, 1992; Crawley, 1993; National Research Council, 1989.
5.2.1. Water soluble
The water soluble vitamins include ascorbic acid, biotin, cobalamin, folates, niacin, pantothenic acid, pyroxidine, riboflavin, and thiamin. A brief summary concerning the functions, deficiency symptoms, food sources and requirements of each vitamin is shown in Table 6.
5.2.2. Fat soluble
The fat soluble vitamins are listed in Table 6 along with a brief summary of their functions, deficiency symptoms, rich food sources and requirements. They include the retinoids (vitamin A), calciferol (vitamin D), the tocopherols and tocotrienols (vitamin E), and phylloquinone and menaquinones (vitamin K). Their bioavailability to humans depends on adequate amounts of lipids and fats in the diet when consumed with plant foods (Combs, 1992).
IMPROVING PLANT FOOD NUTRITIONAL QUALITY AND THE NUTRIENT OUTPUT OF AGRICULTURE SYSTEMS
There are numerous ways in which agriculture can contribute to improving the nutritional quality of plant foods and nutrient output of agricultural systems. However, this requires that agricultural researchers understand i) the importance of such action to society and human health, ii) how they might contribute, and iii) what nutrients are of greatest concern to the nutrition community. Further, government policies must reflect support for such action, and consumers must understand the importance of a diverse and balanced diet to their health, productivity, and well being. Increasing consumer knowledge about the impact of poor nutrition on livelihood, educational attainment, employment opportunities, and health should provide a stimulus to increase the demand for better nutritional quality and diversity of foods. Increased consumer demand for improved products and for more diversity of products available in the marketplace could motivate farmers to produce more nutritious and diverse agricultural products.
There are a number of ways in which agriculture can contribute significantly to finding sustainable solutions to micronutrient malnutrition. Some of these are discussed below. What is currently lacking, however, is the resolve of the agricultural community, the nutrition community, public health officials, private industry, and government policy makers to use agriculture as a primary means in alleviating micronutrient malnutrition. Hopefully, through communications, such as this, it will become abundantly clear to the world's leaders that agriculture holds the paramount means by which sustainable solutions to micronutrient malnutrition can be found. Unless ways are found that will allow agriculture to provide these essential nutrients consistently to all, developing nations will continue to be plagued with "hidden hunger" with all of its unacceptable ramifications for human life.
6.1. Cultural Practices
Cultural practices used by agriculturalists can affect the nutrient output of agricultural systems. However, current agricultural practices are almost always directed at maximizing production while minimizing costs. Recently, in some nations, preserving the environment is becoming a more important objective of agriculture (i.e. "sustainable" agricultural goals). Maximizing nutrient output of farming systems has never been a purport of either agriculture or of public policy. Yet, scientific knowledge is available that could greatly improve the micronutrient output of farming systems, and the available micronutrient content of the food crops produced. The debilitating effects of micronutrient malnutrition on people and societies and its current magnitude in developing nations certainly testifies to the need to consider doing so now. The following discussion briefly presents some examples of how some cultural and agronomic practices could be used to enhance the micronutrient output garnered from farming systems.
6.1.1. Fertilizers and soil amendments
Both macronutrient fertilizers containing N, P, K, and S, and certain micronutrient fertilizers containing, for example Zn, Ni, and Se, can have significant effects on the accumulation of micronutrients in edible plant products (Allaway, 1986; Grunes and Allaway, 1985). Other micronutrient fertilizers have very little if any effect on the amount of the micronutrient accumulated in edible seeds and grains when they are applied to soils or when used as foliar sprays (Welch, 1986). This is especially true for those micronutrient elements with limited phloem-sap mobility such as Fe, B, V and Cr. Some examples of the effects of fertilizer practices on the micronutrient concentrations in edible plant parts are given below. For more detailed information concerning the effects of fertilization practices on micronutrient accumulation in plant foods refer to: Allaway, 1975; Grunes and Allaway, 1985; Karmas and Harris, 1988; Nagy and Wardowski, 1988; Salunkhe and Desai, 1988; Welch, 1997a.
Excessive N fertilizers can adversely affect the accumulation of vitamin C in various vegetable crops such as lettuce (Lactuca sativa L.), beets (Beta vulgaris cicla L.), kale (Brassica oleracea acephala DC.), endive (Cychorium endivia L.),brussels-sprouts (Brassica oleracea gemmifera DC.) by as much as 26% (cited in Salunkhe and Desai, 1988). Interestingly, increasing the amount of K fertilizer supplied to these crops significantly increased their vitamin C content from about 8 to 20% depending on the species.
The concentration of -carotene in carrot [Daucus carota subsp. carota sativus (Hoffm.) Arcang.] roots increased at first harvest in response to increases in the N supplied from 113 mg -carotene 100 g-1 root in those plants supplied 0.3 g N per pot to 126 mg -carotene 100 g-1 root dry weight (about 12% increase) for plants treated with 2.4 g N per pot (Habben, 1972 cited in Salunkhe and Desai, 1988). By the third harvest the increase in -carotene level resulting from increasing N supply was only about 7 %, but the late harvest resulted in an increase in the level of -carotene even in the lowest N treatment from 113 mg -carotene 100 g-1 to 136 -carotene mg 100 g-1 demonstrating a large effect of harvest date on -carotene content of carrot root.
Macronutrient treatments can also influence the concentration of -carotene and other micronutrients in carrots (Welch, 1997a). Vereecke, in 1979 (cited in Salunkhe and Desai, 1988) reported results of studies concerning the effects of combined N, P, K and Mg fertilizers on -carotene, Fe, Mn, Zn and Cu in carrot. Treatments containing N, P and Mg increased the accumulation of -carotene by 42%. Adding K to the fertilizer treatments increased the -carotene by 27% over control plants not receiving K. Removal of Mg from the combined fertilizers lowered the increase in -carotene from 42% to 30%. Apparently, Mg was required for maximum -carotene production in carrots furnished adequate N, P, and K. Vereecke also reported the effects of these treatments on Fe, Mn, Zn and Cu in carrot leaves at two harvest dates. Large effects were found on the Mn and Fe content of the leaves, but the other micronutrients determined were not greatly affected by fertilizer treatments. Combined treatments with N, P, K and Mg increased Fe and Mn concentrations at a late harvest by 20% (from 194 µg Fe g-1 to 234 µg Fe g-1 dry wt.) and 43% (56 µg Mn g-1 to 136 µg Mn g-1 dry wt.), respectively.
The vitamin C concentration in fruits is also affected by macronutrient fertilizers. As with vegetable crops, excessive N fertilization has been reported to reduce vitamin C concentration in the fruits of several species including oranges (Citrus aurantium L.), lemons (Citrus limon L.), mandarins (Citrus reticulata Blanco), cantaloupe (Cucumis melo L.), and apple (Malus domestica Borsh.). Also, higher rates of K fertilization are associated with greater concentration of vitamin C in fruits (Nagy and Wardowski, 1988). Apparently, the effects of Zn, Mg, Mn, and Cu fertilization on increasing vitamin C concentration in citrus fruits is limited to soils that are deficient in these elements. Supplying more of these elements then is required for optimum yield does not increase vitamin C level in the fruit further.
For certain essential micronutrient elements (e.g., Zn, Ni, and Se), increasing their supply to food crops can result in significant increases in their concentrations in edible plant products. For example, increasing the supply of Zn to pea plants (Pisum sativum L.) at levels in excess of that required for maximum yield has been shown to increase the concentration of bioavailable Zn in pea seeds (Peck et al., 1980, Welch, House and Allaway, 1974) (see Figure 7). Further increasing the supply of Zn and Se to wheat (Triticum aestivum L.) (House and Welch, 1989) also increased the Zn and Se concentrations in the mature grain. This has also been shown for navy beans (Phaseolus vulgaris L.) as well as other crops (Moraghan, 1994; Peck, Grunes and Welch, 1980; Welch et al., 1974). For iron, providing more to plants then required to sustain growth does little to further increase the Fe in edible seeds and grains (for example, see Welch and Van Campen, 1975).
The accumulation of micronutrient elements in seeds and grains is controlled by a number of processes including root-cell uptake, root-shoot transfer, and the ability of leaf tissues to load these nutrients into the vascular phloem elements which are ultimately responsible for delivering these nutrients to developing seeds and grains via the phloem sap (Welch, 1986). Phloem loading and unloading of these nutrients is tightly control by poorly understood hemostatic mechanisms in the plant and further research should be carried out to understand these processes if we are to significantly increase certain micronutrient elements, such as Fe, in staple seeds and grains (Welch, 1995).
Soil amendments are frequently used by farmers to adjust soil pH and to enhance plant growth properties of soils. Using lime (CaCO3) raises soil pH permitting acid-intolerant legume species to grow in soils that would otherwise be too acidic for their growth. It is also used to supply Ca to plants. However, adding lime depresses the uptake of Zn, Cu, Fe, and Co, and increases the uptake of Se and Mo by plants. A high soil-pH favors the oxidation of reduced forms of Se such as Se2- and SeO32 to the more soluble and plant-available SeO42 anion.
Gypsum (CaSO4) and S are used to decrease the pH of alkaline soils as well as to provide S for plant uptake and to ameliorate high-Na alkali soils. Using gypsum on alkaline soils could increase plant-available Fe, Mn, Zn, Cu, and Co by decreasing the alkaline soil pH.
The use of farm-yard manures and other forms of organic matter can also change plant-available micronutrients by changing both the physical and biological characteristics of the soil. In many circumstances these changes improve soil physical structure and water holding capacity, resulting in more extensive root development and enhanced soil microflora and fauna activity. All of these can affect available micronutrient levels in soil for plants (Stevenson, 1991; Stevenson, 1994). However, very few controlled experiments have been performed to determine which types of organic matter practices significantly enhance or depress the levels of micronutrients in edible portions of major food crops. More research should be carried out to understand the impact of various types of organic matter on crop nutritional quality.
6.1.2. Variety selection
Using micronutrient-dense staple food crop varieties in cropping systems is one approach that could be used to increase the micronutrient output of farming systems albeit this approach has never been used to date (Bouis, 1996; Combs et al., 1996; Graham and Welch, 1996). There is substantial evidence in the literature that plant traits for micronutrient efficiency and high micronutrient content of edible parts do exist for various plant species (Gerloff and Gabelman, 1983; Graham and Welch, 1996). However, until recently there has been no systematic survey of staple plant food genomes for these types of traits. However, currently, there is such an effort underway for surveying the world genomes of rice (Oryza sativa L.), wheat (Triticum aestivum L. and T. durum Desf.), maize (Zea mays L.), beans (Phaseolus vulgaris L.) and cassava (Manihot esculenta Crantz) for high micronutrient density traits (Bouis, 1996; Graham and Welch, 1996). This effort is administered by one of the Consultative Group on International Agricultural Research (CGIAR) centers, the International Food Policy Research Institute (IFPRI) in Washington, D. C. in cooperation with several other CGIAR centers including Centro Internacional de Agricultura Tropical (CIAT), Centro Internacional de Mejoramiento de Maiz y Trigo (CIMMYT), and the International Rice Research Institute (IRRI), and in collaboration with the Department of Plant Sciences, Waite Campus, University of Adelaide, and the U.S.D.A.-A.R.S., U.S. Plant, Soil and Nutrition Laboratory at Cornell University, Ithaca, NY.
This survey of the staple plant food genomes for micronutrient efficiencies and accumulation ability was based on the premise that increasing the concentrations of micronutrients in staple plant foods through traditional plant breeding techniques would be the most efficient and cost effective means to target micronutrients to people most at risk of developing micronutrient malnutrition, i.e., poor women, infants and children. In the past, most interventions that employ micronutrient supplements or micronutrient food fortification programs have proved not to be sustainable and usually do not effectively reach all the people most at risk. Furthermore, these types of interventions are relatively expensive and require sophisticated infrastructures for their creation, management and maintenance and to assure compliance. The cost of breeding plants with traits that result in significant accumulations of micronutrients in edible portions of staple foods would be a one time cost. Once achieved, these traits can be passed on in breeding programs for future varietal generations and transferred globally to all nations with relatively little additional effort or expense. Thus, this approach to improved micronutrient nutrition is sustainable and cost effective (Bouis, 1996; Graham and Welch, 1996).
Currently, the CGIAR-sponsored screening project for micronutrient-dense staples focuses on three micronutrients, Fe, Zn and provitamin A carotenoids in five staple plant foods (i.e., rice, wheat, maize, beans and cassava) because these foods feed most of the world's poor and because deficiencies of these micronutrients are known to affect vast numbers of people in developing countries. This plant breeding approach could be expanded to include other nutritionally limiting micronutrients such as Se, Cu, vitamin E, vitamin C, folic acid, and other essential trace elements and vitamins if deemed necessary in the future.
Recent results of the current effort to identify micronutrient dense staple plant food genotypes are encouraging. For example, Figure 8 presents the results of an experiment in which 24 genotypes of beans (Phaseolus vulgaris L.), selected by CIAT for their variability in seed-Fe concentrations, were grown in radiolabeled nutrient solutions and subsequently fed to Fe-depleted rats to determine the bioavailability of Fe in the beans. The mature beans harvested varied in Fe concentrations from about 50 µg g-1 to 160 µg g-1 dry weight depending on the genotype. Thus, there was about a three-fold range in Fe concentration in the genotypes studied which demonstrates that beans can be substantially enriched in bioavailable seed-Fe via genetic selection.
There was no relationship between the Fe concentration in the beans and Fe bioavailability to rats fed the beans. This is important in that it demonstrates that increasing the concentration of Fe in bean seeds would be of value in supplying more bioavailable iron to humans even though beans can contain high levels of certain antinutrients such as phytic acid and tannins (polyphenols) that are known to reduce dietary Fe bioavailability under certain circumstances (Lott, 1984; McDonald et al., 1996; Welch and House, 1984).
There is also a large variation in the concentration of Fe and Zn accumulated in the grain of different populations of wheat. As shown in Table 8, different wheat species and genotypes within these species can vary greatly in grain-Fe and grain-Zn concentrations ranging from 24 to 93 µg g-1 for Fe and 27 to 143 µg g-1 for Zn. Thus, it seems feasible for wheat breeders to select for high-Fe and high-Zn density traits in breeding programs. However, further research is needed to determine if the processed edible portions of high-Fe and high-Zn wheat grain (i.e., after milling operations) still retain enriched levels of Fe and Zn, and if enriched levels of Fe and Zn in wheat grain are bioavailable to humans.
Scientists at IRRI recently reported some very exciting news concerning the development of new high yielding rice lines having increased Fe concentrations in their grain. These lines were the direct result of the collaborative survey program administered by IFPRI to identify high grain- Fe genotypes of rice. Rice from different lines grown at the same location during the same season were screened for their grain-Fe concentrations. It became apparent that aromatic rice lines were enriched in Fe and Zn when compared to non-aromatic, high yielding rice varieties (see Figure 9). Scientists at IRRI were able to cross certain aromatic lines with high yielding lines to produce the first high grain-Fe, high yielding rice line. Efforts are now underway to determine if the Fe in the enriched rice grain is bioavailable to humans and if the enriched Fe in the grain remains after post harvest processing (i.e., milling, polishing and parboiling) . If so, then it will not be long before the world will have a new tool to use against Fe deficiency in people dependent on rice for their sustenance.
Selecting micronutrient dense food crops and cultivating micronutrient-dense staple plant food varieties should be a major goal of agriculturalists in developing countries where micronutrient deficiencies among people are common. Certainly, the scientific evidence is sufficient to warrant such an effort. Additionally, this type of agricultural system approach should become a primary instrument used by nutritionists and government policy makers in designing interventions that address micronutrient malnutrition. Doing so, would provide all societies and all people access to nutritionally balanced diets via sustainable food-based system approaches.
6.1.3. Crop management
Crop management is another tool that can be used to improve the micronutrient output of farming systems. For example, using certain legume crops in rotation with cereal crops can result in substantial increases in the concentration of Zn in cereal grain in areas where soil-Zn is currently limiting wheat production (see the Ph. D. thesis of Holloway, 1997).
The selection of crops to avoid micronutrient deficiencies in animals has long been practiced. For instance, in large areas of the United States, soils contain too little Co to meet grazing animal requirements when the livestock are dependent on grasses for feed. However, mixing legumes (that accumulate significantly more Co then grasses) with grasses in pastures is an effective way to supply adequate levels of Co to the grazing animals (Allaway, 1986). Such practices as using pulses in cereal rotations could contribute substantially to increasing the micronutrient output of farming systems in developing countries. This is not only because it could increase the amount of certain micronutrient metals in cereal grains following pulses, but also it would increase the dietary supply of the more micronutrient-rich pulses for local, regional and national markets, thus lowering the cost to consumers and potentially increasing the consumption of these important micronutrient sources by people at risk of developing micronutrient deficiencies.
6.1.4. Indigenous and traditional food crops of high nutritive value
Within many developing nations, certain indigenous food crops are being displaced and lost as important nutritional components of traditional diets. For instance, in Africa during the last few centuries traditional grains, such as African rice (Oryza glaberrima Steudel), Fonio (Digitaria exilis Stapf and D. iburua Stapf), Tef (Eragrostis tef Trotter), pearl millet (Pennisetum glaucum R. Br.), and sorghum (Sorghum bicolor Moench) have been superseded by high yielding cereals (e.g., rice and wheat) introduced and promoted by agricultural experts from developed nations (National Research Council, 1996). The production of many of these traditional crops has decreased even further because of importation of and subsidies paid for millions of tons of wheat, rice and maize that are sold at lower prices. Many traditional crops are much richer sources of micronutrients than the introduced cereal crops that are displacing them as, for example, comparing finger millet to maize (see Figure 10).
Increasing the supply of vegetables to people in many nations would also help reduce the numbers afflicted with micronutrient malnutrition. The Asian Vegetable Research and Development Center (AVRDC) in Taiwan has recommended that people eat 73 kg of vegetables per person per year to satisfy their micronutrient requirements. Figure 11 shows the average per capita vegetable supplies of several nations in Asia. With the exception of China, South Korea and Taiwan, none of the countries depicted in Figure 11 produce enough vegetables to meet this recommendation.
Designing cropping systems for maximum nutrient output to improve nutrition and health should become an integral part of agriculture's goals and government policies. Additionally, ways must be found to increase dietary diversity among disadvantaged people. This would substantially reduce the risk of micronutrient malnutrition to the most at risk people globally. Furthermore, any increase in the production of more micronutrient-rich foods (micronutrient-dense food crops, livestock, dairy or fish) could contribute greatly to finding sustainable solutions to micronutrient malnutrition. Given these axioms, selecting cropping systems not only for their production potential, but also for their ability to supply needed dietary sources of bioavailable micronutrients should become a goal of all nations if we are to meet the laudable objectives of better health and prosperity for all.
6.2. Molecular alterations of plant genes to improve micronutrient supplies
Modern molecular biological techniques could be used to genetically alter food plants with increased bioavailable concentrations of micronutrients in edible portions. However, this requires detailed knowledge of various physiological and biochemical processes in plants. Several homoeostatic plant processes must be altered to allow for increased accumulation of micronutrients in edible plant products. These processes include increased uptake of micronutrient elements by roots, increased translocation of micronutrient elements form roots to shoots, increased re-mobilization of micronutrients from shoots to reproductive organs, and increased deposition of micronutrients in edible portions of food crops. For vitamins, increased biosynthesis and accumulation of these micronutrients must be expressed in edible organs of the plant. All of these potential genetic modifications must be done without negatively affecting crop yields, crop quality or consumer acceptability. Finally, the micronutrients must be in forms that are bioavailable to the people that eat the plant foods in meals that contain numerous other interacting dietary components. Figure 12 displays some general features of the genotypical mechanisms that play roles in nutrient efficiency processes in plants. These types of mechanisms could be genetically altered to increase the accumulation of micronutrients in plants if the genes that control these plant functions were identified. The discussion below outlines ways that these tasks could be accomplished if sufficient resources were available to do so.
6.2.1. Increasing efficiency of micronutrient uptake
The mineral nutrition of plants is under genetic control and the mechanisms by which plants accumulate micronutrient elements are under genetic regulation. Unfortunately, plant breeders normally have not taken advantage of micronutrient element efficiency traits to enhance the ability of major food crops to absorb micronutrient elements from micronutrient-poor soils. Commonly, breeders have used their most productive soils to breed high yielding, disease and stress resistant crops. These fertile soils contain ample available sources of micronutrient elements for crops. Because breeders normally use these highly fertile soils for their selections, they may have inadvertently lost micronutrient element efficiency traits during their genetic selection for high yielding traits because there were no selection pressures to preserve such traits in the selection process. However, there is ample evidence to show that these traits do exist in plant genomes and that they can be selected for in breeding programs. Graham and his colleagues have published extensively on this subject for various micronutrients (e.g., Zn, Mn and Cu) and for several cereal crops including wheat, oats and barely (Graham, 1984; Graham, 1988a; Graham, 1988b; Graham et al., 1992; Graham and Welch, 1996; Rengel and Graham, 1995b; Rengel and Graham, 1995a). Others have also reported that there is significant genetic diversity for micronutrient uptake traits that can be used to enrich micronutrient elements in plants grown on micronutrient-poor soils (Clark, 1982; Marschner, 1995). Selecting for the ability to accumulate more micronutrient elements from nutrient-poor soils is the first step in breeding for micronutrient-dense staple food crops.
6.2.2. Increasing translocation, re-mobilization and deposition of micronutrients
The second step in increasing the density of micronutrient in staple foods involves altering the genes that control the translocation of root-accumulated micronutrient elements to shoots. Here also there is sufficient evidence to suggest that this can be done by genetic selection, but more research is required to more fully delineate the processes and genes involved (Welch, 1986; Welch, 1995).
Once more micronutrients are accumulated in plant shoots, they must be re-translocated out of stems and leaves to reproductive organs before they can be deposited in developing seeds and grains. Re-translocation requires the loading of micronutrient elements from source tissues into vascular phloem elements and the phloem sap, long distance transport within the phloem sap, and unloading of micronutrient elements out of the phloem sap and into sink sites within reproductive organs. The mechanisms that control these processes in plants are not known with any certainty. Further research is needed to determine what these mechanisms are and what genes are responsible for their construction and their regulation.
Knowing how micronutrients are stored and in what forms they are occur in edible seeds and grains is also an important consideration (see discussion below) for increasing the bioavailable content of micronutrients in edible plant parts. Here also, very little is known about this aspect of micronutrients in plants and much more research should be directed at increasing our knowledge in this area (Welch, 1986; Welch, 1995).
6.2.3. Improving the bioavailability of micronutrients in plant foods
Increasing the concentrations of micronutrients in edible plant foods is only the first step in making these foods richer sources of micronutrients for humans. This is because not all of the micronutrients in plant foods are bioavailable to humans that eat these foods. Plant foods can contain substances (i.e., antinutrients) that interfere with the absorption or utilization of these nutrients in humans. Table 9 list some common antinutrients that are found in many staple plant foods.
Plant breeders could breed for genotypes that contain lower concentrations of antinutrients or molecular biologists could alter plant genes in ways that reduce or even eliminate antinutrients from plant foods. However, doing so is not without risk, and should be done with caution because many antinutrients are major plant metabolites that may play important roles in plant metabolism and in plant resistance to crop pests or pathogens. Additionally, some of the antinutrients may play important beneficial roles in human diets by acting as anticarcinogens or by promoting health in other ways such as in decreasing the risk of heart disease or diabetes. Thus, plant breeders and molecular biologists should be aware of the possible negative consequences of changing antinutrients in major plant foods before they attempt to alter food crops in this fashion (Graham and Welch, 1996).
Other substances can promote the bioavailability of micronutrients in plant foods to humans even in the presence of antinutrients in those foods. Some substances that promote Fe and Zn bioavailability are listed in Table 10. Many of these compounds are normal plant metabolites and only small changes in their concentration may have significant effects on the bioavailability of micronutrients. Therefore, it is highly recommended that plant breeders and molecular biologists closely scrutinize this strategy of increasing promoter substances in food crops when attempting to improve food crops as sources of micronutrients for people (Welch, 1993).
6.2.4. Increasing the accumulation of vitamins in edible parts of food crops
Currently, much of the research on increasing vitamins in food crops has been directed at the provitamin A carotenoids in vegetable crops (Camara et al.,1992; Schuch et al.,1996; Simon, 1992). In the past some research was also performed to determine the variability of ascorbic acid (vitamin C) in various fruits and vegetables and the environmental factors that influence ascorbic acid concentrations in fruits such as tomato (Salunkhe and Deshpande, 1991). Unfortunately, our knowledge of the biosynthetic and regulatory processes that control the accumulation of other vitamins in food crops is extremely limited and much more research will need to be preformed before significant progress can be made in genetically altering staple food crops as sources of vitamins for humans. However, current molecular biological techniques are available that would allow for rapid genetic alterations of plant foods in ways that would increase vitamins in these foods once the biosynthetic pathways and their genetic regulation are understood (New York Academy of Sciences, 1996).
Established biochemical approaches to delineate vitamin metabolism in plants has depended on the purification of enzymes and determinations of their activity in vitro. These approaches are being displaced by molecular genetic techniques using mutants of plants [such as Arabidopsis thaliana (L.) Heynh.] and the expression of plant genes in heterologous systems (Simon, 1992; Camara et al., 1992). These model plant systems are currently being used to understand vitamin metabolism in plants, such as Arabidopsis mutants, to screen for altered expression of carotenoids (Cunningham et al., 1996; Pogson et al., 1996). This has enabled the cloning of genes for various biosynthetic enzymes, and also has demonstrated the extent to which provitamin A carotenoids can be altered within the plant, without impaired growth and function of the plant. A second approach includes using genes identified in non-plant organisms, such as yeast or bacteria, to identify homologous genes in plants for vitamin biosynthetic pathways of interest (Burkhardt et al., 1997; Yamano et al., 1994). Once the plant genes have been cloned, their expression can be manipulated to determine the consequences to plant growth and the extent to which various vitamins can be increased (Phillips, 1993; Comai, 1993; Watson, 1995).
7. FOOD-BASED SYSTEM APPROACHES TO HEALTHIER AND HAPPIER LIVES
All people are dependent on food systems of varying complexity to supply the nutrients required to sustain human life. Yet, food systems have developed with no attention given to their ability to provide all the nutrients required to support good health, productive lives and well being. Inadequate nutrition lowers the quality of life for billions of disadvantaged people globally especially in many developing countries. Food systems should be exploited in ways that will improve their potential to provide adequate nutrient output to offer the poor a reasonable chance for healthier and more productive lives (Combs et al., 1997b; Welch et al., 1997b). For this to occur, there will have to be major changes in the way agriculturalists, nutritionists, public health officials and policy makers view the role of agriculture in national nutrition, health, and development goals. Nations need to recognize that agriculture is the primary provider of nutrients for all people, and therefore, agricultural systems should be the primary tool used in programs to eliminate micronutrient malnutrition globally. Not doing so, will result in increased malnutrition worldwide, and sustainable solutions to micronutrient malnutrition will never be realized.
A new agenda for food and agricultural development is needed in all nations to meet the increasing nutritional demands of an expanding global population. This should be directed at increasing the production of nutritionally adequate food supplies in ways that protect biological, socio-economic and political realities, thus ensuring sustainability. For this to ensue, agriculture, nutrition, and national development goals must be viewed in the larger context of their inherent interrelationships; that is from a holistic food systems perspective (see Figure 13).
In order to focus food systems on developing sustainable solutions to micronutrient malnutrition globally, it will be necessary to address this crisis using system approaches that confront the problem in multidisciplinary ways taking comprehensive views of both the goals and the means to achieve the goals. While the agricultural sector normally has viewed success in terms of production goals alone, food system views would expand that view to include the impact of agricultural production on human nutrition and health outcomes.
Food system perspectives can facilitate the development of food-based intervention strategies for assailing micronutrient malnutrition (Combs et al., 1996). The food system concept includes all factors related to the production, acquisition and utilization of food. It considers food systems to be varied, complex, multi-component networks with multiple inputs (labor, capital, knowledge, seed source, etc.) and multiple outcomes with health and well-being of people being the major focus of the system. Food systems include various activities nested within several subsystems encompassing production (land use and tenure, soil and crop management, plant breeding, varietal selection, livestock breeding and management, and harvesting), acquisition (e.g., food processing, transportation, storage, packaging and marketing), and utilization (i.e., food preparation, processing and cooking, household food decision-making, food preferences, and access to health care, sanitation, energy, and nutritional knowledge). These subsystems interact to varying degrees within different contexts covering the biophysical, social, economic, public health and policy environments. In this type of holistic conceptualization of poor nutrition, food system failures are viewed as responsible for micronutrient malnutrition. Food system approaches to micronutrient malnutrition would be directed at identifying root causes and then at developing sustainable solutions to the failures identified.
Using food system approaches to prevent micronutrient malnutrition will require enunciation of the values and foci that would direct such approaches. These values and foci include:
Food system approaches should be focused on staple plant foods (e.g., rice, wheat, maize, beans and cassava) because these are the most important dietary sources of micronutrients for the most at risk people. Three approaches are most appropriate to achieve this end:
To promote these paradigm reforms for agriculture and nutrition, institutional changes will be needed to promote more effective transdisciplinary linkages between agricultural and health professionals and to facilitate holistic views of agriculture, health and development. Barriers to such change, inadvertently created by narrow disciplinary orientations, must be removed by developing programs that focus on problems and that reward those engaged in transdiciplinary collaborations. The ultimate goals of the agriculture, nutrition, health and development communities must be viewed as fundamentally linked to each other, and programs must reflect this vision. Sustainable means to these aspirations are necessary in order to provide humans with a foreseeable future that will reflect happier and healthier lives for all. This cannot be achieved without having a population that is better nourished and healthier, more vigorous and productive in every way, not only in ways that enhance labor productivity, but also for mental creativity, social accordance, and civic life. Food system perspectives capture this view of life: people are both the means and the end, and their well being is the dominant motivating force and ultimate evaluative criterion for any program. Therefore, forging close linkages between the agriculture, nutrition, and health arenas requires the adoption of food system approaches if we are to foster sustainable means to end the conundrum of micronutrient malnutrition (Combs et al. 1997b; Combs et al., 1996; Welch et al., 1997b).
8. REFERENCES
Abdulla, M., Parr, R.M. & Iyengar, G.V. 1993 Trace element requirements, intake and recommendations. Progress in Clinical and Biological Research 380: 311-328.
Allaway, W. H. 1975 The effects of soils and fertilizers on human and animal nutrition. USDA-ARS Agriculture Information Bulletin No. 378., U.S. Government Printing Office, Washington, D.C..
Allaway, W. H. 1986 Soil-plant-animal and human interrelationships in trace element nutrition. In: Trace Elements in Human and Animal Nutrition (Mertz, W., ed.), Academic Press, Orlando, San Diego, New York, Austin, London, Montreal, sydeney, Tokyo, Toronto pp. 465-488.
Anonymous 1986 Trace Elements in Human and Animal Nutrition. Vol. 2, Ed.5, Academic Press, Inc., San Diego, New York. 499 pp.
Anonymous 1987 Trace Elements in Human and Animal Nutrition. Vol. 1, Ed.5, Academic Press, Inc., San Diego, New York.480 pp.
Anonymous 1992 International Conference on Nutrition: World Declaration and Plant of Action. International Conference on Nutrition, Dec. 5-11, 1992, Rome, Italy. Food and Agricultural Organization and World Health Organization, United Nations, Rome. pp. 1-42.
Anonymous 1993 Preventing micronutrient deficiencies: food abundance and diversity are fundamental. Food Nutr Agr 7: 8-17.
Anonymous 1994 The Challenge of Dietary Deficiencies of Vitamins and Minerals. In: Enriching Lives: Overcoming Vitamin and Mineral Malnutrition in Developing Countries World Bank, Washington D.C. pp. 6-13.
Anonymous 1995 Progress report on the implementation of the ICN world plan of action for nutrition. FAO Conference, Twenty-eighth Session, Oct. 20- Nov. 2, 1995, Rome, Italy. Document C 95/INF/18. Food and Agricultural Organization, United Nations, Rome. pp. 1-14.
Anonymous 1996 Food security and nutrition. World Food Summit Technical Background Document WFS 96/TECH/9. Food and Agricultural Organization, United Nations, Rome. pp. 1-45.
Barefield, E. 1996 Osteoporosis-related hip fractures cost $13 billion to $18 billion yearly. Food/Review Jan.-Apr.: 31-36.
Beaton, G.H., Martorell, R., L'Abbé, M.R., Edmonston, B., McCabe, G., Ross, A.C. & Harvey, B. 1993 Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. SCN News 9: 17-23.
Behrman, J.R. 1993 The Economic Rationale for Investing in Nutrition in Developing Countries. Monograph. US Agency for International Development / VITAL, Washington, D.C. pp. 1-32.
Bouis, H. 1996 Enrichment of food staples through plant breeding: a new strategy for fighting micronutrient malnutrition. Nutrition Reviews 54: 131-137.
Brown, M.L. 1990 Present Knowledge in Nutrition. International Life Sciences Institute Nutrition Foundation, Washington, D.C. pp. 1-532.
Burkhardt, P.K., Beyer, P., Wuenn, J., Kloeti, A., Armstrong, G.A., Schledz, M., Von Lintig, J.& Potrykus, L. 1997 Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J 11:1071-1078.
Buyckx, M. 1993 The international community's commitment to combating micronutrient deficiencies. Food Nutr Agr 7: 2-7.
Camara, B., Schantz, R. & Moneger, R. 1992 Enzymology and genetic regulation of carotenoid biosynthesis in plants. In: Biotechnology and Nutrition (Bills, D.D. and Kung, S., eds.), Butterworth-Heinemann, Boston pp. 301-314.
Chapin, R.E., Ku, W.W., Kenney, M.A., McCoy, H., Gladen, B., Wine, R.N., Wilson, R. & Elwell, M.R. 1997 The effects of dietary boron on bone strength in rats. Fundamental and Applied Toxicology 35: 205-215.
Clark, L.C., Combs, G.F., Jr., Turnbull, B.W., Slate, E.H., Chalker, D.K., Chow, J., Davis, L.S., Glover, R.A., Graham, G.F., Gross, E.G., Krongrad, A., Lesher, J.L., Jr., Park, H.K., Sanders, B.B., Jr., Smith, C.L. & Taylor, J.R. 1996 Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin a randomized controlled trial - A randomized controlled trial. J Am Med Assoc 276: 1957-1963.
Clark, R.B. 1982 Plant genotype differences to uptake, translocation, accumulation, and use of mineral elements. In: Genetic specificity of mineral nutrition of plants, Scientific Assemblies Vol. 13 (Saric, M.R., ed.), Serbian Academy of Sciences and Arts, Belgrade pp. 41-55.
Comai, L. 1993 Impact of plant genetic engineering on foods and nutrition. Ann Rev Nutr 191-215.
Combs, G.F., Duxbury, J.M. & Welch, R.M. 1997a Food systems for imporved health: linking agricultural production and human nutrition. European Journal Biochemistry 51: S32-S33
Combs, G. F., Welch, R. M., and Duxbury, J. M. 1997 Sustainable food system approaches to improving nutrition and health In: Proceedings of an International Conference on Agricultural Production and Nutrition, March 19-21, 1997, Tufts University, Medford, MA (Lockeretz, W., ed.), Tufts University School of Nutrition and Policy, Boston. pp. 1-9.
Combs, G.F., Jr. 1992 The Vitamins: Fundamental Aspects in Nutrition and Health, Academic Press, San Diego, CA. pp. 1-528.
Combs, G.F., Jr., Welch, R.M., Duxbury, J.M., Uphoff, N.T. & Nesheim, M.C. 1996 Food-Based Approaches to Preventing Micronutrient Malnutrition: an International Research Agenda, Cornell International Institute for Food, Agriculture, and Development, Cornell University, Ithaca, NY. 68 pp.
Crawley, H. 1993 Natural occurrence of vitamins in food. In: The Technology of Vitamins in Food (Ottaway, P.B., ed.), Blackie Academic & Professional, Glasgow, England pp. 19-41.
Culver, B.D. & Hubbard, S.A. 1996 Inorganic boron health effects in humans: An aid to risk assessment and clinical judgment. Journal of Trace Elements in Experimental Medicine 9: 175-184.
Cunningham, R.X. Jr., Pogson, B., Sun, Z., McDonald, K.A., Dellapenna, D. & Gantt, E. 1996 Functional analysis of the beta and epsilon lycopene cycalse enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. Plant Cell 8:1613-1626.
Doesthale, Y.G., Devara, S., Rao, S. & Belavady, B. 1979 Effect of milling on mineral and trace element composition of raw and parboiled rice. J. Sci. Food Agric. 30: 40-46.
Endre, L., Beck, F.W.J. & Prasad, A.S. 1990 The role of zinc in human health. Journal of Trace Elements in Experimental Medicine 3: 337-375.
Foster, H.D. 1993 The iodine-selenium connection: Its possible roles in intelligence, cretinism, sudden infant death syndrome, breast cancer and multiple sclerosis. Medical Hypotheses 40: 61-65.
Frazao, E. 1996 The American diet: a costly health problem. FoodReview Jan./April: 2-6.
Garcia-Casal, M.N., Layrisse, M., Solano, L., Arguello, F., Llovera, D., Ramírez, J., Leets, I. & Tropper, E. 1998 Vitamin A and b-carotene can improve nonheme iron absorption from rice, wheat and corn by humans. J Nutr 128: 646-650.
Ge, K. & Yang, G. 1993 The epidemiology of selenium deficiency in the etiological study of endemic diseases in China. Am J Clin Nutri 57 Suppl.: 259S-263S.
Gerloff, G.C. & Gabelman, W.H. 1983 Genetic basis of inorganic plant nutrition. In: Inorganic Plant Nutrition (Lauchli, A. and Bieleski, R.L., eds.),Springer-Verlag, Berlin, Heidelberg, New York, Tokyo pp. 453-480.
Gibson, R.S. 1994 Zinc nutrition in developing countries. Nutr Res Rev 7: 151-173.
Gibson, R.S., Heywood, A., Yaman, C., Sohlström, A., Thompson, L.U. & Heywood, P. 1991 Growth in children from the Wosera subdistrict, Papua New Guinea, in relation to energy and protein intakes and zinc status. Am J Clin Nutri 53: 782-789.
Goldenberg,R.L. 1995 The effects of zinc supplementation on pregnancy outcome. J Am Med Assoc 274:463-468.
Gordon, N. 1997 Nutrition and cognitive function. Brain Dev. 19: 165-170.
Graham, R.D. 1984 Breeding for nutritional characteristics in cereals. Adv Plant Nutr 1: 57-102.
Graham, R.D. 1988a Development of wheats with enhanced nutrient efficiency: progress and potential. In: Wheat Production Constraints in Tropical Environments. Proceedings of the International Conference, Maj, Thiland 19-23 January, 1987 (Klatt, A.R., ed.), CIMMYT, Mexico DF, Mexico pp. 305-320.
Graham, R.D. 1988b Genotypic differences in tolerance to manganese deficiency. In: Manganese in Soils and Plants (Graham, R.D., Hannam, R.J. and Uren, N.C., eds.),Kluwer Academic Publishers, Dordrecht, The Netherlands pp. 216-276.
Graham, R.D., Ascher, J.S. & Hynes, S.C. 1992 Selecting zinc-efficient cereal genotypes for soils of low zinc status. Plant Soil 146: 241-250.
Graham, R.D. & Welch, R.M. 1996 Breeding for staple-food crops with high micronutrient density. Agricultural Strategies for Micronutrients Working Paper 3, International Food Policy Research Institute, Washington, D.C. pp. 1-72.
Grunes, D.L. & Allaway, W.H. 1985 Nutritional quality of plants in relation to fertilizer use. In: Fertilizer Technology and Use. (Engelstad, O.P., ed.), Soil Science Society of America, Madison, WI pp. 589-619.
Hambidge, K.M. 1992 Zinc and diarrhea. Acta Paediatrica Scandinavica 81 Suppl. 381: 82-86.
Hambidge, K.M. 1997 Zinc deficiency in young children. Am J Clin Nutri 65: 160-161.
Hetzel, B.S. 1990 Iodine deficiency: an international public health problem. In: Present Knowledge in Nutrition (Brown, M.L., ed.), International Life Sciences Institute, Nutrition Foundation, Washington, D.C. pp. 308-313.
Hofbauer, L.C., Spitzweg, C., Magerstädt, R.A. & Heufelder, A.E. 1997 Selenium-induced thyroid dysfunction. Postgrad. Med. J. 73: 103-104.
Holland, B., Welch, A.A., Unwin, I.D., Buss, D.H., Paul, A.A. & Southgate, D.A.T. 1991 McCance and Widdowson's The Composition of Foods, Ed. 5, The Royal Society of Chemistry, Letchworth, UK. 462 pp.
Holloway, R. 1997 Zinc as a subsoil nutrient for cereals. Ph. D. Thesis, University of Adelaide, South Australia..
House, W.A. & Welch, R.M. 1989 Bioavailability of and interactions between zinc and selenium in rats fed wheat grain intrinsically labeled with 65Zn and 75Se. J Nutr 119: 916-921.
Islam, S. & Tori, T.H. 1998 The invisible adversary. Star Magazine Jan. 9: 4-10.
Karmas, E. & Harris, R.S. 1988 Nutritional Evaluation of Food Processing, Ed. 3, Avi Book, Van Nostrand Reinhold Co., New York. 786 pp.
Kim, E.-S., Noh, S.K. & Koo, S.I. 1998 Marginal zinc deficiency lowers the lymphatic absorption of a-tocopherol in rats. J Nutr 128: 265-270.
Larsen, T. 1997 Erythrocyte membrane enzymes as indicators of zinc status. In: Trace Elements in Man and Animals - 9. Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals (Fischer, P.W.F., L'Abbé, M.R., Cockell, K.A. and Gibson, R.S., eds.), National Research Council of Canada, Ottawa pp. 105-106.
Li, R., Chen, X., Yan, H., Deurenberg, P., Garby, L. & Hautvast, J.G.A.J. 1994 Functional consequences of iron supplementation in iron- deficient female cotton mill workers in Beijing, China. Am. J. Clin. Nutr. 59: 908-913.
Looker, A.C., Dallman, P.R., Carroll, M.D. & Gunter, E.W. 1997 Prevalence of iron deficiency in the United States. J Am Med Assoc 277: 973-976.
Lott, J.N.A. 1984 Accumulation of seed reserves of phosphorus and other minerals. In: Seed Physiology. Vol. I. Development (Murray, D.R., ed.), Academic Press, Sydney, New York, London pp. 139-166.
Maberly, G.F., Trowbridge, F.L., Yip, R., Sullivan, K.M. & West, C.E. 1994 Programs against micronutrient malnutrition: ending hidden hunger. Annu Rev Public Health 15: 277-301.
Marschner, H. 1995 Mineral Nutrition of Higher Plants, Ed. 2, Academic Press, London. 889 pp.
Mason, J.B. & Garcia, M. 1993 Micronutrient deficiency - the global situation. SCN News 9: 11-16.
McDonald, M., Mila, I. & Scalbert, A. 1996 Precipitation of metal ions by plant polyphenols: Optimal conditions and origin of precipitation. J Agric Food Chem 44: 599-606.
McGuire, J. 1993 Addressing micronutrient malnutrition. SCN News 9: 1-10.
Moraghan, J.T. 1994 Accumulation of zinc, phosphorus, and magnesium by navy bean seed. J Plant Nutr 17: 1111-1125.
Nagy, S. & Wardowski, W.F. 1988 Effects of agricultural practices, handling, processing, and storage on fruits. In: Nutritional Evaluation of Food Processing (Karmas, E. and Harris, R.S., eds.),Avi Book, Van Nostrand Reinhold Co., New York pp. 73-100.
National Research Council. 1989 Recommended Dietary Allowances, National Academy Press, Washington, D.C. pp. 1-285.
National Research Council. 1996 Lost Crops of Africa, Vol. 1, Grains, National Academy Press, Washington, D.C. 383 pp.
New York Academy of Sciences. 1996 Engineering Plants for Commercial Products and Applications. Annals of the New York Academy of Sciences Vol. 792, The New York Academy of Sciences, New York. 181 pp.
Nielsen, F.H. 1990 New essential trace elements for the life sciences. Biol Trace Element Res 26-27: 599-611.
Nielsen, F.H. 1992 Nutritional requirements for boron, silicon, vanadium, nickel, and arsenic: current knowledge and speculation. FASEB J 5: 2661-2667.
Nielsen, F.H. 1993 Ultratrace elements of possible importance for human health: An update. Progress in Clinical and Biological Research 380: 355-376.
Nielsen, F.H. 1994 Biochemical and physiologic consequences of boron deprivation in humans. Environ. Health Perspect. 102 Suppl. 7: 59-63.
Nielsen, F.H. 1996 Evidence for the nutritional essentiality of boron. Journal of Trace Elements in Experimental Medicine 9: 215-229.
Nielsen, F.H. 1997 Beyond copper, iodine, selenium and zinc: other elements that will be found important in human nutrition by the year 2000. In: Trace Elements in Man and Animals - 9. Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals (Fischer, P.W.F., L'Abbé, M.R., Cockell, K.A. and Gibson, R.S., eds.), NRC Research Press, Ottawa. pp. 653-655.
Peck, N., Grunes, D.L., Welch, R.M. & MacConald, G.E. 1980 Nutritional quality of vegetable crops as affected by phosphorus and zinc fertilizers. Agron J 72: 528-534.
Penland, J.G. 1997 Trace elements, brain function and behavior: effects of zinc and boron. In: Trace Elements in Man and Animals - 9. Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals (Fischer, P.W.F., L'Abbé, M.R., Cockell, K.A. and Gibson, R.S., eds.),National Research Council of Canada, Ottawa pp. 213-216.
Phillips, R.L. 1993 Plant genetics: out with the old, in with the new? Am J Clin Nutri 58: 259s-263s.
Pogson, B., McDonald, K.A., Truong, M., Britton, G. & DellaPenna 1996 Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8:1627-1639.
Prasad, A.S. 1993 Essential and toxic trace elements in human health and disease: An update. Marginal deficiency of zinc and immunological effects. Progress in Clinical and Biological Research 380: 1-22.
Prasad, A.S. 1996 Zinc deficiency in women, infants and children. Journal of the American College of Nutrition 15: 113-120.
Ramalingaswami, V. 1995 New global perspectives on overcoming malnutrition. Am J Clin Nutr 61: 259-263.
Reinhold, J.G. 1988 Problems in mineral nutrition: a global perspective. In: Trace Minerals in Foods (Smith, K.T., ed.), Marcel Dekker, Inc., New York and Basel pp. 1-55.
Rengel, Z. & Graham, R.D. 1995a Wheat genotypes differ in Zn efficiency when grown in chelate- buffered nutrient solution. Plant Soil 176: 307-316.
Rengel, Z. & Graham, R.D. 1995b Wheat genotypes differ in Zn efficiency when grown in chelate- buffered nutrient solution .2. Nutrient uptake. Plant Soil 176: 317-324.
Rivera, J.A., Ruel, M.T., Santizo, M.C. & Lönnerdal, B. 1998 Zinc supplementation improves the growth of stunted rural Guatemalan infants. J Nutr 128: 556-562.
Salunkhe, D.K. & Desai, B.B. 1988 Effects of agricultural practices, handling, processing, and stroage on vegetables. In: Nutritional Evaluation of Food Processing (Karmas, E. and Harris, R.S., eds.),Avi Book, Van Nostrand Reinhold Co., New York pp. 23-71.
Salunkhe, D.K. & Deshpande, S.S. 1991 Foods of Plant Origin: Production, Technology, and Human Nutrition, AVI Book, Van Nostran Reinhold, New York. 501 pp.
Sanghvi, T.G. 1996 Economic Rationale for Investing in Micronutrient Programs. A Policy Brief Based on New Analyses, Office of Nutrition, Bureau for Research and Development, United States Agency for International Development, Washington, D.C. pp. 1-12.
Sazawal, S., Black, R.E., Bhan, M.K., Jalla, S., Bhandari, N., Sinha, A. & Majumdar, S. 1996 Zinc supplementation reduces the incidence of persistent diarrhea and dysentery among low socioeconomic children in India. J Nutr 126: 443-450.
Schuch, W., Drake, R., Römer, S. & Bramley, P.M. 1996 Manipulating carotenoids in transgenic plants. Annals of the New York Academy of Sciences 792: 13-19.
Shrimpton, R. 1993 Zinc deficiency - is it widespread but under-recognized? SCN News 9: 24-27.
Simon, P.W. 1992 Genetic improvement of vegetable carotene content. In: Biotechnology and Nutrition. Proceedings of the Third International Symposium (Bills, D.D. and Kung, S.-D., eds.),Butterworth-Heinemann, Boston pp. 291-314.
Stevenson, F.J. 1991 Organic matter-micronutrient reactions in soil. In: Micronutrients in Agriculture, Ed. 2 (Mortvedt, J.J., Cox, F.R., Shuman, L.M. and Welch, R.M., eds.), Soil Sci. Soc. Am., Madison, WI pp. 145-186.
Stevenson, F.J. 1994 Humus chemistry: Genesis, Composition, Reactions, Ed.2, Wiley, New York.496 pp.
Underwood, B.A. 1998 From research to global realtity: the micronutrient story. J Nutr 128:145-151.
U.S. Department of Health and Human Services. 1988 The Surgeon General's Report on Nutrition and Health. Public Health Service Publication No. 88-50210, Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 727 pp.
U.S. Department of Health and Human Services 1990 Healthy People 2000. Nutr Today November/December: 29-39.
United Nations ACCSN. 1992 Second Report on the World Nutrition Situation. Vol. I. Global and Regional Results. United Nations Administrative Committee on Coordination, Subcommittee on Nutrition, Geneva, Switzerland. pp. 1-80.
Walter, T., Peirano, P. & Roncagliolo, M. 1997 Effect of iron deficiency anemia on cognitive skills and neuromaturation in infancy and childhood. In: Trace Elements in Man and Animals - 9. Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals (Fischer, P.W.F., L'Abbé, M.R., Cockell, K.A. and Gibson, R.S., eds.),National Research Council of Canada, Ottawa pp. 217-219.
Watson, J.M. 1995 Improving the nutritional and functional qualities of plant food through genetic engineering. Proc Nutr Soc Aust 19: 73-79.
Welch, R.M. 1986 Effects of nutrient deficiencies on seed production and quality. Adv Plant Nutr 2: 205-247.
Welch, R.M. 1993 Zinc concentrations and forms in plants for humans and animals. In: Zinc in Soils and Plants (Robson, A.D., ed.), Kluwer Academic Publishers, Dordrecht, Boston, London. pp. 183-195.
Welch, R.M. 1995 Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 14: 49-82.
Welch, R.M. 1997 Agronomic problems related to provitamin A carotenoid-rich plants. European Journal of Clinical Nutrition 51: S34-S38
Welch, R.M., Combs, G.F., Jr. & Duxbury, J.M. 1997 Toward a "Greener" revolution. Issues in Science and Technology 14: 50-58.
Welch, R.M. & House, W.A. 1984 Factors affecting the bioavailability of mineral nutrients in plant foods. In: Crops as Sources of Nutrients for Humans. (Welch, R.M. and Gabelman, W.H., eds.), American Society of Agronomy, Madison, WI pp. 37-54.
Welch, R.M., House, W.A. & Allaway, W.H. 1974 Availability of zinc from pea seeds to rats. J Nutr 104: 733-740.
Welch, R.M. & Van Campen, D.R. 1975 Iron availability to rats from soybeans. J Nutr105: 253-256.
Wenlock, R.W. 1992 Trace element requirements and DRVs. Food Chemistry 43: 225-231.
Wharton, B.A. 1992 Trace element deficiency in man: Classifications and methods of assessment. Food Chemistry 43: 219-224.
World Health Organization. 1995 Global Prevalence of Vitamin A Deficiency. WHO Micronutrient Deficiency Information System Working Paper No. 2, World Health Organization, Geneva, Switzerland. pp. 1-116.
World Health Organization. 1996 Trace elements in human nutrition and health, World Health Organization, Geneva. pp. 1-343.
Yi, M. & Xia, Y. 1997 Effects of selenium repletion and depletion on selenium status and thyroid hormone metabolism in rats with mild iodine deficiency. In: Trace Elements in Man and Animals -9. Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals (Fischer, P.W.F., L'Abbé, M.R., Cockell, K.A. and Gibson, R.S., eds.),NRC Research Press, Ottawa, Canada pp. 621-623.
Yip, R. 1997 The challenge of improving iron nutrition: limitations and potentials of major intervention approaches. European Journal of Clinical Nutrition 51: S16-S24
Yamano, S., Ishii, T., Nakagawa, M., Ikenaga, H., & Misawa, N. 1994 Metabolic engineering for production of beta-carotene and lycopene in Saccharomyces cerevisiae. Biosci Biotech Biochem 58:1112-1114.
Zook, G.E., Greene, F.E. & Morris, E.R. 1970 IV. Distribution of Mn, Cu, Ni, Zn, Mg, Pb, Sn, Cd, Cr and Se as determined by atomic absorption spectrography and calorimetry. Cereal Science 47: 720-731.
Table 1. Micronutrient element concentration comparisons between whole cereal grains and pulse seeds.
| Plant Food | Fe | Zn | Mn | Cu | Mo | Cr | Ni |
| µg g-1, dry weight | |||||||
| Brown rice 1 | 22 | 14 | 11 | 2.4 | 0.78 | 0.088 | - |
| Soft wheat 2 | - | 22 | 35 | 4.5 | - | 0.370 | 0.31 |
| Mung bean 3 | 87 | 41 | 14 | 13.0 | 3.20 | 0.251 | 2.04 |
| Black gram 3 | 139 | 36 | 19 | 7.9 | 0.16 | 0.530 | 3.43 |
| Cowpea 3 | 67 | 45 | 16 | 6.3 | 1.47 | 0.272 | 3.44 |
| Soybean 4 | 97 | 43 | 26 | 15.5 | - | - | - |
| Red kidney bean 5 | 64 | 30 | 12 | 6.8 | - | - | - |
1 Data from Doesthale et al., 1979.
2 Data from Zook et al., 1970.
3 Unpublished data provided by R. M. Welch.
4 Data from Holland et al., 1991.
5 Data from Holland et al., 1991.
Table 2. Effect of milling and polishing on micronutrient concentrations in rice grain; data from Salunkhe and Deshpande, 1991.
| Micronutrient 1 | Whole Brown Rice | Polished Rice | % Reduction |
| Iron (mg/kg) | 30 | 10 | 67 |
| Copper (mg/kg) | 3.3 | 2.9 | 12 |
| Manganese (mg/kg) | 17.6 | 10.9 | 62 |
| Zinc (mg/kg) | 18 | 13 | 30 |
| Thiamin (mg/kg) | 3.4 | 0.7 | 80 |
| Riboflavin (mg/kg) | 0.5 | 0.3 | 40 |
| Niacin (mg/kg) | 47 | 16 | 66 |
| Vitamin B 6 (mg/kg) | 6.2 | 0.4 | 94 |
| Folic Acid (µg/kg) | 200 | 160 | 20 |
| Pantothenic Acid (mg/kg) | 20 | 10 | 50 |
| Biotin (µg/kg) | 120 | 50 | 58 |
| Vitamin E (IU/kg) | 20 | 10 | 50 |
1 Dry weight basis.
Table 3. World regions (World Health Organization) where populations are affected by or are at risk of developing iron, iodine or vitamin A deficiencies; data from Anonymous, 1994.
WHO World Region |
Iodine deficiency 1 |
Vitamin A deficiency 1 |
Iron deficiency 1 | ||
| At risk | Affected 2 | At risk | Affected 3 | Iron deficient or anemic 4 | |
| Africa | 150 | 39 | 18 | 1.3 | 206 |
| Americas | 55 | 30 | 2 | 0.1 | 94 |
| S. & S.E. Asia | 280 | 100 | 138 | 10.0 | 616 |
| Europe | 82 | 14 | &SHY; | &SHY; | 27 |
| E. Mediterranean | 33 | 12 | 13 | 1.0 | 149 |
| W. Pacific & China | 405 | 30 | 19 | 1.4 | 1,058 |
| Total | 1,005 | 225 | 190 | 13.8 | 2,150 |
1 Numbers in millions of people.
2 Affected by goiter.
3 Affected by xerophthalmia (a general term for all eye signs of severe vitamin A deficiency including blindness).
4 Causes of anemia, other then iron deficiency, often include malaria, intestinal parasites, other nutrient deficiencies, such as folate and vitamin B 12 and genetically determined hemoglobinopathies, such as thalasemia. However, at least half of the anemia worldwide is thought to be directly the result of dietary iron deficiency (United Nations ACCSN, 1992).
Table 4. Percent prevalence of iron deficiency and anemia in certain population groups within the United States; data from U.S.Department of Health and Human Services, 1990.
| Population Group | Percent Affected |
| Base line (1976-1980) | |
| Low-income children (1-2 years old) | 21 |
| Low-income children (3-4 years old) | 10 |
| Low-income women (child-bearing age) | 8 |
| Base line (1983-1985) | |
| Alaska Native children (1-5 years old) | 22-28 |
| Black, low income pregnant women | 41 |
1 Anemia was used as an indicator of iron deficiency.
Table 5. Essential trace elements: examples of some deficiency implications, functions, estimated dietary needs and rich food sources1.
| Element | Deficiency and Function(s) in Animals and Humans | Human Dietary Need 2 ;Rich Food Sources |
| Arsenic | impaired fertility and increased perinatal mortality; depressed growth; conversion of methionine to its metabolites; methylation of biomolecules | 12 µg d -1 (?); fish, grain and cereal products |
| Boron | impaired Ca utilization in bone; more severe signs of vitamin D related rickets; decreased apparent absorption of Ca, Mg, and P; impaired mental functions in older women and men (>45 years old); cis-hydroxyl reactions with biomolecules; cell membrane integrity | 0.5 - 1.0 mg d -1 (?); noncitrus fruits, leafy vegetables, nuts, and pulses |
| Chromium | impaired glucose tolerance; impaired growth; elevated serum cholesterol and triglycerides; increased incidence of aortic plaques; corneal lesions; decreased fertility and sperm count; potentiates insulin action | 33 µg d -1 (?); processed meats, whole grain products, pulses, and spices |
| Copper | hypochromic anemia; neutropenia; hypopigmentation of hair and skin; impaired bone formation with skeletal fragility and osteoporosis; vascular abnormalities; steely hair; metal cofactor in numerous metalloenzymes, e.g., cytochrome oxidase, caeruloplasmin, superoxide dismutase, etc. | 1.5 - 3.0 mg d -1 ; organ meats, seafood, nuts and seeds; in drinking water from Cu plumbing in urban areas |
| Fluorine | status as an essential trace element debated; beneficial element because of its effects on dental health | 1.5 - 4.0 mg d -1 ; tea, marine fish consumed with bones |
| Iodine | wide spectrum of diseases including severe cretinism with mental retardation; enlarged thyroid (goiter); essential constituent of the thyroid hormones | 150 µg d -1 ; seafood, iodized table salt; milk; I concentrations in plant foods vary greatly depending on various environmental factors including the geochemical environment, fertilizer, food processing and feeding practices |
| Iron | iron deficiency erythropoiesis with low iron stores and with work capacity performance impaired; iron deficiency anemia with reduced hemoglobin levels and small red blood cells; impaired immune function; apathy; short attention span; reduced learning ability; constituent of hemoglobin, myoglobin and a number of enzymes | 15 mg d -1 ; meats, eggs, vegetables and iron-fortified cereals |
| Manganese | poor reproductive performance; growth retardation; congenital malformations; abnormal bone and cartilage formation; impaired glucose tolerance; metal activator of many enzymes, e.g., decarboxylases, hydrolases, kinases, and transferases; constituent of pyruvate carboxylase and superoxide dismutase in mitochondria | 2.0 - 5.0 mg d -1 ; whole grain and cereal products, fruits and vegetables, tea |
| Molybdenum | retarded weight gain; decreased food consumption; impaired reproduction; shortened life expectancy; neurological dysfunction; dislocated ocular lenses, mental retardation; cofactor (molybdopterin) in sulfite oxidase and xanthine dehydrogenase | 75 - 250 µg d -1 ; milk, beans, breads and cereals |
| Nickel | depressed growth and reproductive performance; impaired functioning and body distribution of several nutrients e.g., Ca, Fe, Zn, vitamin B 12 ; cofactor for an enzyme that affects amino acids and odd-chained fatty acids derived from the propionate metabolic pathways | <100 µg d -1 ; chocolate, nuts, dried beans, peas and grains |
| Selenium | endemic cardiomyopathy (Keshan disease); white muscle disease; endemic osteoarthoropathy (Kashin-Beck disease) with enlargement and deformity of the joints; liver necrosis; exudative diathesis; pancreatic atrophy; growth depression; depressed activity of 5'-deiodinase enzymes that produce triiodothyronine (T 3 ) from thyroxine (T 4 ); impaired immune response to viral infections; anticarcenogenic activity; essential component of glutathione peroxidase and "selenoprotein-P" | 55 - 70 µg d -1 ; seafood, organ meats; meats; cereal grains grown on Se-rich soils; Brazil nuts; Se concentrations in plant foods can vary greatly depending on the available Se content of the soil where grown and the plant species grown |
| Silicon | depressed collagen content in bone with skull structure abnormalities; long bone abnormalities; decreased articular cartilage, water, hexosamine, and collagen; decreased levels of Ca, Mg, and P in tibias and skulls under Ca deficiency conditions | 5 - 20 µg d -1 (?); unrefined grains, cereal products; root and tuber crops |
| Vanadium | death proceeded by convulsions; skeletal deformities; increased thyroid weight; participates in oxidation of halide ions and/or the phosphorylation of receptor proteins | <10 µg d -1 (?); shellfish, mushrooms, black pepper, dill seed |
| Zinc | loss of appetite; growth retardation; skin changes; immunological abnormalities; difficulty in parturition; teratogensis, hypogonadism; dwarfism; impaired wound healing; suboptimal growth, poor appetite, and impaired taste acuity in infants and children; diarrhea; impaired immune function; constituent of numerous enzymes; cellular membrane stability function | 15 mg d -1 ; animal products especially red meats, cheese, legume seeds and pulses |
1 Sources of information included: National Research Council, 1989; Nielsen, 1992; World Health Organization, 1996.
2 Reported daily allowances are for adult men.
Table 6. Essential vitamins: examples of some deficiency implications, functions, estimated dietary needs and rich food sources 1 .
| Deficiency and Function(s) in Animals and Humans | Human Dietary Need 2 ; Rich Food Sources | |
| Water soluble | ||
Ascorbic acid (vitamin C) |
scurvy (weakening of collagenous substances); widespread capillary hemorrhaging; plays roles in leukocyte and macrophage functions; immune responses; allergic reactions; wound healing; substrate for hydroxylations involving molecular oxygen (e.g., proline and lysine involvement in collagen synthesis); antioxidant; promoter of non-heme Fe(III) bioavailability | 60 mg d-1; citrus fruits; potatoes; peppers; broccoli; spinach; tomatoes, green leafy vegetables |
| Biotin | urinary excretion of organic acids; skin rash; hair loss; anorexia; nausea; vomiting; glossitis; pallor; mental depression; alopecia; dry scaly dermatitis; > serum cholesterol and bile pigments; integral part of enzymes involved in carboxyl unit transport and carbon dioxide fixation (e.g., integral part of pyruvate carboxylase and acetyl-coenzyme A carboxylase) | 30-100 µg d-1; liver, egg yolk, soy flour, yeast |
Cobalamin (vitamin B12) |
macrocytic, megaloblasic anemia; demyelinaton of spinal cord, brain, optic and peripheral nerves; soure tongue; weakness; neuropsychiatric symptoms in absence of anemia in the elderly; Co is an integral component of methylcobalamin or 5'-deoxyadenosylcobalamin; catalyzes transmethylation from a folic acid cofactor to homosysteine to form methionine; involved in nucleic acid synthesis in single C transfer reactions; degradation of some amino acids and odd-chain fatty acids | 2.0 µg d-1; animal product accumulation from bacteria |
Folates (folic acid) |
impaired cell division; alterations in protein synthesis; hypersegmentation of cells; megaloblastic bone marrow; macrocytic anemia; function as coenzymes transporting single C fragments from one molecule to another in amino acid metabolism and nucleic acid synthesis | 200 µg d-1; liver, yeast, leafy vegetables, legumes, some fruits |
Niacin (nicotinamide) |
Pellagra including dermatitis, diarrhea, inflammation of the mucous membranes, dementia; component of 2 coenzymes, nicotinamide adenine dinucleotide & nicotinamide dinucleotide phosphate, that participate in numerous metabolic processes (e.g., glycolysis, fatty acid metabolism, tissue respiration) | 19 mg niacin eq. d-1 (1 niacin eq. = 60 mg dietary tryptophan); meats, foods rich in tryptophan that can be converted to niacin such as milk and eggs |
| Pantothenic acid | reduced growth rate; infertility; abortion; increased rates of neonatal death; skin, hair, pigmentation abnormalities; neuromuscular disorders; gastrointestinal abnormalities; adrenal cortical failure; sudden death; "burning feet" syndrome; components of coenzyme A and the acyl carrier protein of fatty acid synthetase for acyl activation and transfer reactions; important in sterol, steroid hormone, porphyrin and acetylcholine synthesis and in acylation reactions | 4-7 mg d-1; animal tissues, whole cereal grains, legumes |
Pyroxidine (vitamin B6) |
epileptiform convulsions; dermatitis; anemia; neurological symptoms and abdominal distress in infants; includes pyridoxine, pyridoxal, and pyridoxamine; converted to pyridoxal phosphate and pyridoxamine phosphate which serve as coenzymes in transformations of amino acids including transamination, decarboxylation and racemization reactions; lipid and nucleic acid metabolism; coenzyme for glycogen phosphorylase | 2 mg d-1; chicken, fish, kidney, liver, pork, eggs, unmilled rice, soy beans, oats, whole wheat, peanuts, walnuts |
| Riboflavin | oral-buccal cavity lesions including cheilosis and angular stomatitis; generalized seborrheic dermatitis; scrotal and vulval skin abnormalities; normocytic anemia; component of two flavin coenzymes, flavin mononucleotide and flavin adenine dinucleotide involved in oxidation and reduction reactions | 1.7 mg d-1; meats, poultry, fish, dairy products, green vegetables (e.g., broccoli, turnip greens, asparagus and spinach) |
| Thiamin | beriberi; symptoms of mental confusion, anorexia, muscular weakness, ataxia, peripheral paralysis, ophthalmoplegia, edema, muscle wasting, tachycardia, and enlarged heart; increased plasma pyruvate levels; impediments of carbohydrate metabolism related to reduced oxidative decarboxylation reactions of -keto acids; reduced thiamin pyrophosphate saturation of erythrocyte transketolase; transketolase activation in the pentose phosphate pathway | 1.5 mg d-1; unrefined cereal gains, brewer's yeast, organ meats, lean cuts of port, legumes, seeds and nuts |
| Fat soluble | ||
Vitamin A (retinoids) |
ocular signs in children (xeropthalmia) including night blindness, conjunctival xerosis, corneal xerosis, ulceration and liquefaction; keratomalacia (partial or total blindness); loss of appetite; hyperkeratosis; increased morbidity; metaplasia and keratinization of epithelial respiratory cells, tract and other organs; required for visual system, growth and development, maintenance of epithelial cellular integrity, immune function, and reproduction | 1000 µg RE d-1 (RE = 1 µg retinol or 6 µg -carotene); liver, fish liver oils, whole milk, eggs, carrots, dark-green leafy vegetables (e.g., spinach) |
Vitamin D (calciferol) |
rickets; impaired bone mineralization; hypocalcemia; secondary hypothyroidism; osteomalacia; prohormone for the regulation of steroid hormone action involving Ca metabolism and Ca-mineral homeostasis; vitamin D3 (cholecalciferol) is synthesized in skin exposed to u.v. light | 5 µg d-1 (as cholecalciferol and 10 µg cholecalciferol = 400 IU vit. D); eggs, fortified foods including milk, butter and margarine |
Vitamin E (tocopherols & tocotrienols) |
reproductive failure; muscular dystrophy; neurological abnormalities; antioxidants for defense against harmful oxidations; protection of polyunsaturated fatty acids and phospholipids from oxidation in cell membranes | 10 mg -TE d-1 (-TE = 1 mg d- tocopherol); common vegetable oils (e.g., soybean, corn , cotton seed and safflower), wheat germ, nuts, green leafy vegetables |
Vitamin K (phylloquinone & menaquinones) |
defective coagulation of blood; required for the synthesis of prothrombin in blood and other proteins in the plasma, bone and kidney that regulate blood clotting; these proteins are involved in Ca ion binding in blood, regulation of bone crystal formation, and possibly some phospholipids synthseis | 80 µg d-1 green leafy vegetables (e.g., spinach, broccoli, brussels sprouts, kale, & turnip greens), lower but significant amounts in milk, meats, eggs, cereals fruits and other vegetables |
1 Sources of information included: Brown, 1990; National Research Council, 1989; Nielsen, 1992; World Health Organization, 1996.
2 Reported daily allowances are for adult men.
Table 7. Effects of increasing Zn and Se supplies to wheat plants grown in nutrient solutions on the concentration of Zn and Se in mature wheat grain and bioavailable amounts of Zn and Se in the grain when fed to Zn-deficient rats in a single meal (data from House and Welch, 1989).
| Zn Supplied | Se Supplied | Zn in Grain | Se in Grain | Bioavailable Zn | Bioavailable Se |
| --------(µM)--------- | ------(µg g-1, dry wt.)------ | ----(µg absorbed from meal)---- | |||
| 1.0 | 0.3 | 8.8 | 0.6 | 5.9 | 0.41 |
| 1.0 | 1.5 | 9.3 | 3.8 | 5.5 | 2.03 |
| 5.0 | 0.3 | 33.0 | 0.6 | 18.7 | 0.42 |
| 5.0 | 1.5 | 36.1 | 4.3 | 15.4 | 1.91 |
Table 8. Variation in Fe and Zn concentrations in mature grain of diverse wheat populations from various world regions (unpublished data furnished by Robin Graham and CIMMYT, 1997).
| Region | Population | Fe (SD)* | Range | Zn (SD) | Range |
| Turkey | 133 landraces (1993) | 51(3.5) | 43-62 | 33 (5.6) | 19-48 |
| Mexico | 1000 diverse germplasm entries | ||||
| 50 Triticum dicoccon (1992) | 62 (17) | 24-93 | 80 (26) | 27-135 | |
| 30 Triticum tauschii (1995) | 76 (10) | 59-99 | 50 (7) | 38-69 | |
| 47 Triticum dicoccoides (1992) | 42 (6) | 27-93 | 68 (27) | 27-143 | |
| Various | Breeder's lines | ||||
| 154 Triticum aestivum (1995) | 42 (6) | 33-73 | 40 (8) | 27-85 | |
| 230 Triticum durum (1995) | 37 (3.5) | 30-47 | 47 (6) | 34-63 | |
| 239 Triticosecale rimpaui (1995) | 33 (3) | 27-57 | 46 (6) | 33-66 | |
*SD = standard deviation
Table 9. Examples of antinutrients in plant foods and common dietary sources.
| Antinutrient | Dietary source |
| Phytin (phytic acid) | legume seeds & cereal grains |
| Fiber | whole cereal grain |
| Tannins (polyphenolics) | tea, coffee, certain bean varieties, sorghum |
| Oxalic acid | spinach leaves, rhubarb |
| Hemagglutinins (lectins) | most legume seeds & wheat grain |
| Heavy metals (Cd, Hg, Pb, Ag) | plant foods from polluted soils |
Table 10. Substances that promote the bioavailability of Fe and/or Zn in staple plant foods and common dietary sources.
| Promoter Substance | Dietary Source |
Organic acids (ascorbate, fumarate, malate, citrate) |
fresh fruits & vegetables |
Certain amino acids (methionine, cystine, histidine, & lysine) |
animal meats, eggs, milk |
Long-chain fatty acids (palmitic acid) |
human milk |
Phytoferritin (Fe-storage protein) |
in plastids of plant cells within various plant organs including leaves, roots and seeds of many species |
-carotene1 (provitamin A carotenoids) |
dark green leafy vegetables, orange vegetables |
Animal meat factors (identities unknown) |
beef, pork, fish, poultry, etc. |
1 García-Casal et al. (1998) recently reported that both vitamin A and -carotene improves nonheme Fe absorption from rice, wheat and corn to humans.
Figure 1. Total population growth trends in the world, Asia, Africa, South America and the United States between 1978 to 1996 (data from FAO, FAOSTAT Database, 1998).

.
Figure 2. Yearly trends in dietary Energy Supplies from
1975 to 1990 in some global regions; data from United Nations ACCSN, 1992
Figure 3. Yearly trends in dietary iron density and
% anemic women (non-pregnant, 15 to 49 years old) in South Asia; data from
United Nations ACCSN (1992). Units on second y-axis are percent of women
with iron deficiency (i.e., having less then 12 g hemoglobin dL1 of blood).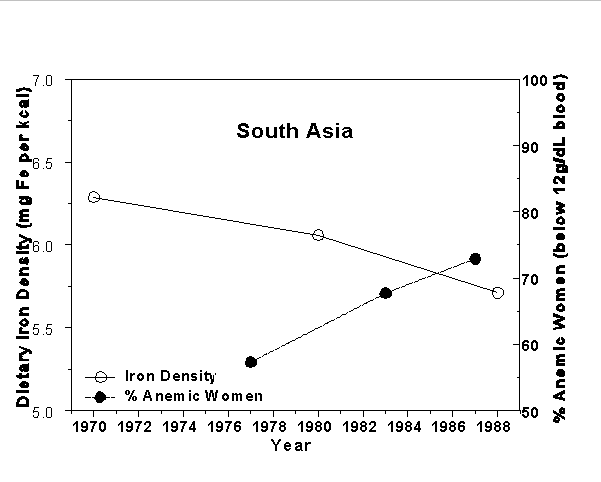
Figure 4. Time trends in total rice, wheat and pulses per capita production in Bangladesh between 1978 to 1996 (data from FAO, FAOSTAT Database, 1998).
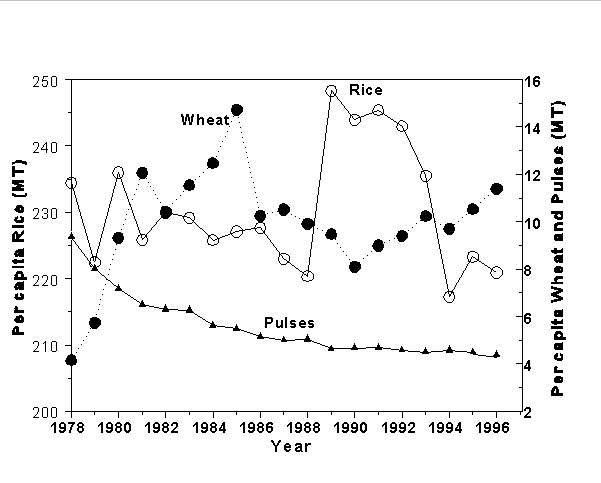
Figure 5. Prevalence of iron, vitamin A and iodine in humans in developing nations; map modified from Sanghvi (1996).
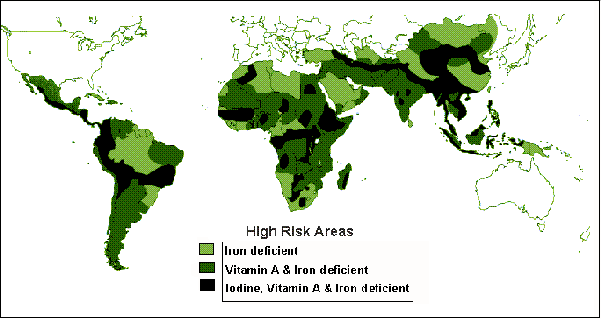
Figure 6. Benefits of improved infant, children and pregnant women nutrition on health, productivity, economic development and societal stability.

Figure 7. Effects of increasing Zn supply to pea (Pisum sativum L.) plants grown in 65 Zn radiolabeled nutrient solutions on Zn concentration and bioavailable Zn in mature pea seeds fed in single meals to Zn-depleted rats (data from Welch et al., 1974).
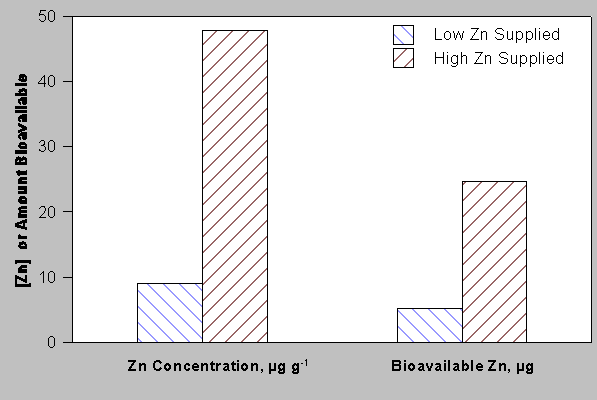
Figure 8. Genetic variability of 24 genotypes of bean (Phaseolus vulgaris L.) seeds in bean-Fe concentration and bean-Fe bioavailability determined in rats fed bean meals prepared from plants grown in nutrient solutions radiolabeled with 59Fe (unpublished data from Welch, House, Beebe and Cheng, 1998). Error bars represent standard error of the mean (n=5).

Figure 9. Concentration (dry wt. bases) of Fe and Zn in grain of select genotypes of aromatic and non-aromatic rice (unpublished data from Robin Graham and IRRI, 1997).
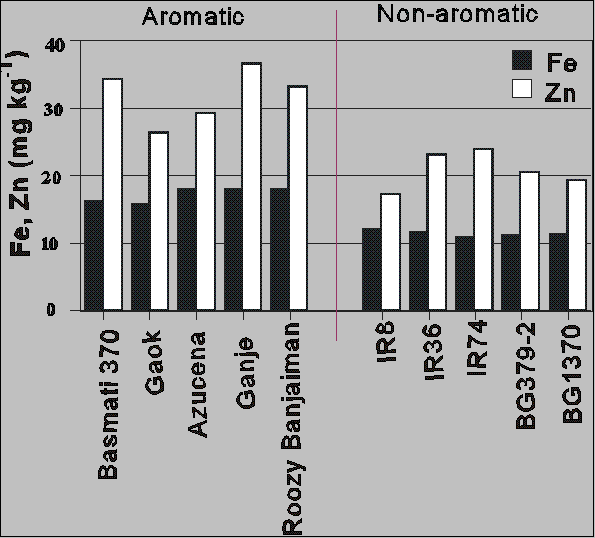
Figure 10. Nutritional quality of finger millet (Eleusine coracana L.) seed compared to maize kernels (Figure modified from National Research Council, NAS, 1996).

Figure 11. Asian nations' vegetable supplies (three year average, 1986-1988). The recommended per person supply of vegetables is 73 kg year-1 to meet micronutrient requirements (modified from unpublished figure developed by Ali et al., Asian Vegetable Research and Development Center, 1994).

Figure 12. Genotypical mechanisms of nutrient efficiency (modified from Marschner, 1995).

Figure 13. Food systems model showing the relationships between food-based approaches to sustainable solutions to micronutrient malnutrition and their impact on human health and well being (from Combs et al., 1996).

1. Corresponding author: Ross M. Welch, U.S. Plant, Soil and Nutrition Laboratory, Tower Road, Ithaca, NY 14853; telephone: 607-255-5434; FAX: 607-255-1132; Email: RMW1@CORNELL.EDU
2. Micronutrients are dietary nutrients, including essential elements and vitamins, that are required by humans in very small amounts. They include at least 14 trace elements (As, B, Cr, Cu, F, I, Fe, Mn, Mo, Ni, Se, Si, V, and Zn) and 13 vitamins ( thiamin, riboflavin, niacin, pantothenic acid, biotin, folic acid, vitamin B6, vitamin B12 vitamin C, vitamin A, vitamin D, vitamin E, and vitamin K).
3. Bioavailability is defined as the amount of a nutrient in a food that is absorbable and physiologically utilizable by the person consuming the meal.
4. Vitamins are organic substances occurring in trace amounts in foods that are essential to human life.